Journal of Peking University (Health Sciences) ›› 2025, Vol. 57 ›› Issue (6): 1024-1031. doi: 10.19723/j.issn.1671-167X.2025.06.003
Previous Articles Next Articles
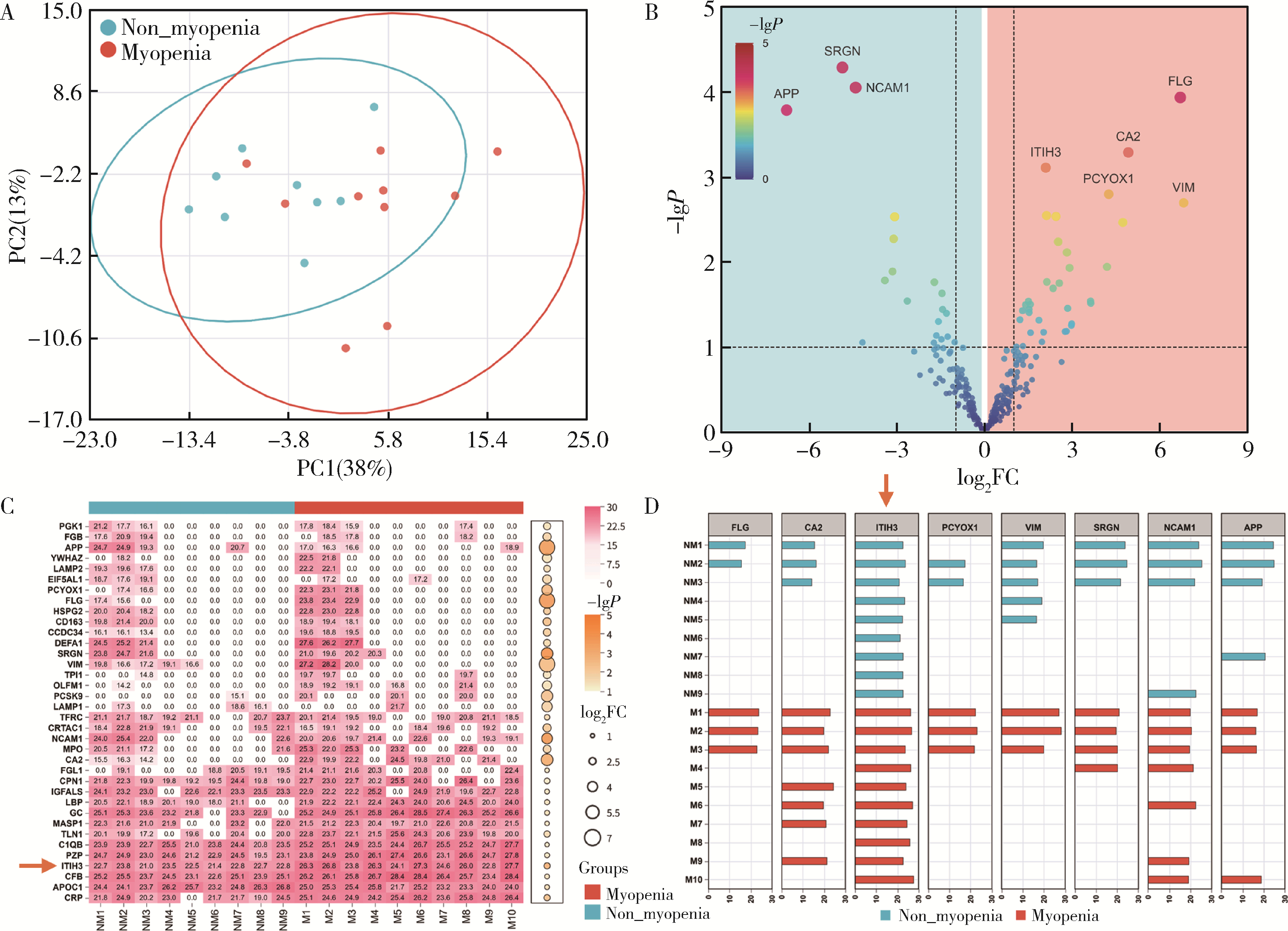
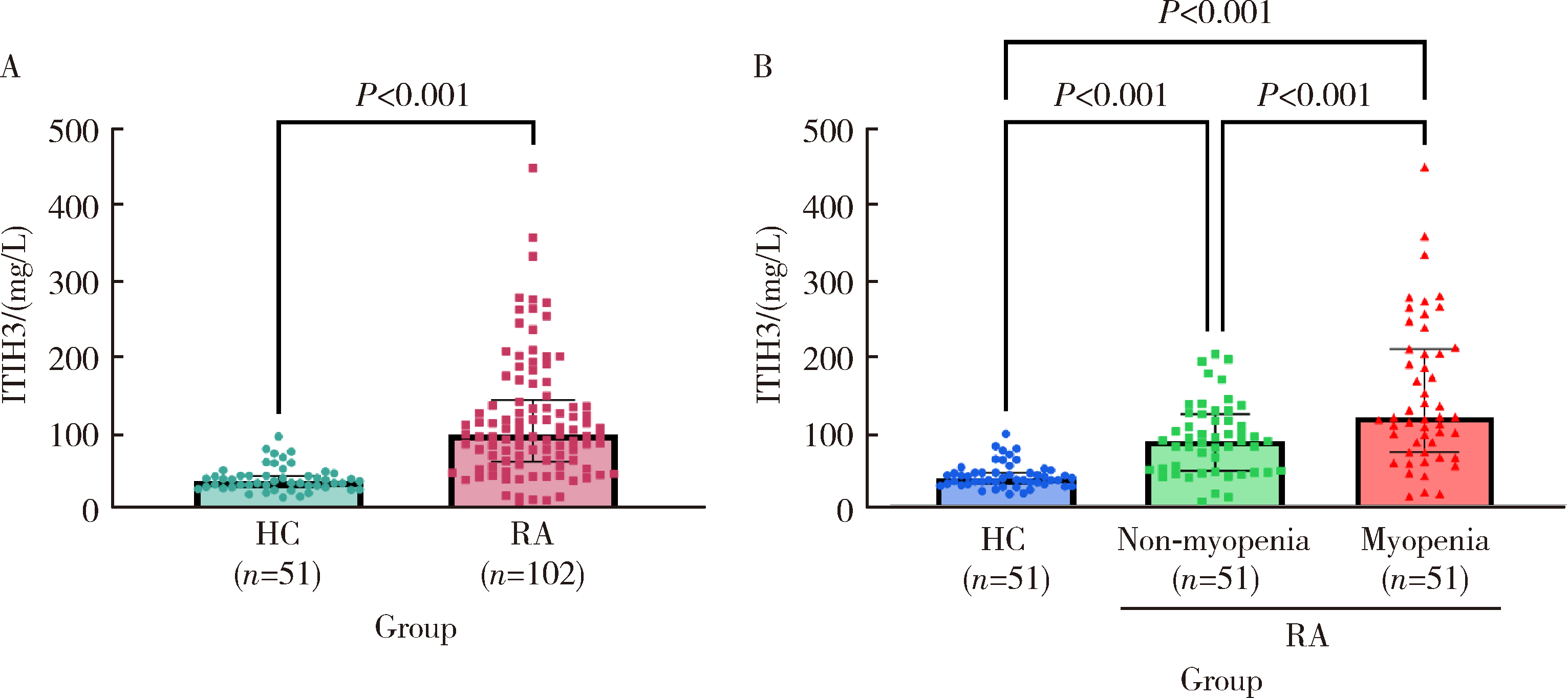
Serum inter-alpha-trypsin inhibitor heavy chain H3 is identified as a potential biomarker for myopenia in patients with rheumatoid arthritis using proteomic profiling
Tao WU1, Jianzi LIN1, Yafeng ZHU2, Jianda MA1, Peiwen JIA1, Lijuan YANG1, jie PAN1, Yaowei ZOU1, Ying YANG1, Ye LU1, Lie DAI1,*( )
)
- 1. Department of Rheumatology and Immunology, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou, 510120, China
2. Basic and Translational Medical Research Center, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou, 510120, China
CLC Number:
- R593.22
| 1 |
中华医学会老年医学分会, 国家老年疾病临床医学研究中心(湘雅医院). 中国肌肉减少症诊疗指南(2024版)[J]. 中华医学杂志, 2025, 105(3): 181- 203.
|
| 2 |
doi: 10.1038/s41413-025-00438-9 |
| 3 |
doi: 10.1007/s13539-011-0025-7 |
| 4 |
doi: 10.1002/jcsm.12381 |
| 5 |
doi: 10.1080/03009742.2020.1842902 |
| 6 |
doi: 10.1038/s41584-023-00921-9 |
| 7 |
doi: 10.1007/s40520-016-0717-0 |
| 8 |
doi: 10.3390/medicina61040551 |
| 9 |
doi: 10.3389/fnut.2022.1007184 |
| 10 |
doi: 10.1177/1759720X20946220 |
| 11 |
国家皮肤与免疫疾病临床医学研究中心(北京协和医院), 中国医师协会风湿免疫专科医师分会, 中国康复医学会风湿免疫病康复专业委员会, 等. 2024中国类风湿关节炎诊疗指南[J]. 中华内科杂志, 2024, 63(11): 1059- 1077.
|
| 12 |
贾霈雯, 杨迎, 邹耀威, 等. 类风湿关节炎患者低肌肉量综合征的临床特征及其对躯体功能的影响[J]. 北京大学学报(医学版), 2024, 56(6): 1009- 1016.
|
| 13 |
doi: 10.1074/jbc.M808560200 |
| 14 |
doi: 10.1074/jbc.M111.324913 |
| 15 |
doi: 10.1186/s12967-025-06563-7 |
| 16 |
doi: 10.3390/cells11081256 |
| 17 |
doi: 10.1007/s00401-024-02754-6 |
| [1] | Zhao XIANG, Li YANG, Jing YANG. Untargeted metabolomics reveals differential serum metabolites and metabolic pathways in patients with primary Sjögren syndrome and thrombocytopenia [J]. Journal of Peking University (Health Sciences), 2025, 57(6): 1042-1050. |
| [2] | Yan DING, Lifang WANG, Chaoran LI, Zhemin LU, Lianjie SHI. Rheumatoid arthritis combined with IgG4-related disease successfully treated with rituximab: A case report [J]. Journal of Peking University (Health Sciences), 2025, 57(6): 1203-1207. |
| [3] | Xiangyu SUN, Chao YUAN, Xinzhu ZHOU, Jing DIAO, Shuguo ZHENG. Application of salivary micro-ecosystem in early prevention and control of oral and systemic diseases [J]. Journal of Peking University (Health Sciences), 2025, 57(5): 859-863. |
| [4] | Congyi YANG, Xiaowen ZHENG, Jingyi CHEN, Jun XU, Feng CHEN, Yang CHEN, Ning CHEN. Protein biomarker screening and functional analysis of salivary exosomes in patients with ulcerative colitis [J]. Journal of Peking University (Health Sciences), 2025, 57(5): 895-902. |
| [5] | Ju YANG, Jing XU, Juhua DAI, Lianjie SHI. Expression of lumican protein in serum of patients with rheumatoid arthritis and its correlation with disease and immune activities [J]. Journal of Peking University (Health Sciences), 2025, 57(5): 911-918. |
| [6] | Lianghua FENG, Lirong HONG, Yujia CHEN, Xueming CAI. Role and mechanism of ubiquitin-specific protease 35 in ferroptosis of rheumatoid arthritis-fibroblast like synoviocytes [J]. Journal of Peking University (Health Sciences), 2025, 57(5): 919-925. |
| [7] | Peiwen JIA, Ying YANG, Yaowei ZOU, Zhiming OUYANG, Jianzi LIN, Jianda MA, Kuimin YANG, Lie DAI. Clinical characteristics of overlapping syndromes of low muscle mass in patients with rheumatoid arthritis and their impact on physical function [J]. Journal of Peking University (Health Sciences), 2024, 56(6): 1009-1016. |
| [8] | Doudou MA, Zhemin LU, Qian GUO, Sha ZHU, Jin GU, Yan DING, Lianjie SHI. Successful treatment of rheumatoid arthritis complicated with myasthenia gravis with low-dose rituximab: A case report [J]. Journal of Peking University (Health Sciences), 2024, 56(6): 1110-1114. |
| [9] | Rui YAN, Dan KE, Yan ZHANG, Li LI, Huanran SU, Wei CHEN, Mingxia SUN, Xiaomin LIU, Liang LUO. Diagnostic significance of serum chemokine CXCL-10 and Krebs von den lungen-6 level in patients with rheumatoid arthritis associated interstitial lung disease [J]. Journal of Peking University (Health Sciences), 2024, 56(6): 956-962. |
| [10] | Liang ZHAO, Chenglong SHI, Ke MA, Jing ZHAO, Xiao WANG, Xiaoyan XING, Wanxing MO, Yirui LIAN, Chao GAO, Yuhui LI. Immunological characteristics of patients with anti-synthetase syndrome overlap with rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2024, 56(6): 972-979. |
| [11] | Yijun HAN, Xiaoli CHEN, Changhong LI, Jinxia ZHAO. Application status of methotrexate in patients with rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2024, 56(6): 994-1000. |
| [12] | Dongwu LIU, Jie CHEN, Mingli GAO, Jing YU. Rheumatoid arthritis with Castleman-like histopathology in lymph nodes: A case report [J]. Journal of Peking University (Health Sciences), 2024, 56(5): 928-931. |
| [13] | Huina HUANG,Jing ZHAO,Xiangge ZHAO,Ziran BAI,Xia LI,Guan WANG. Regulatory effect of lactate on peripheral blood CD4+ T cell subsets in patients with rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2024, 56(3): 519-525. |
| [14] | Xiaofei TANG,Yonghong LI,Qiuling DING,Zhuo SUN,Yang ZHANG,Yumei WANG,Meiyi TIAN,Jian LIU. Incidence and risk factors of deep vein thrombosis in patients with rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2024, 56(2): 279-283. |
| [15] | Xue ZOU,Xiao-juan BAI,Li-qing ZHANG. Effectiveness of tofacitinib combined with iguratimod in the treatment of difficult-to-treat moderate-to-severe rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 1013-1021. |
|
||