Journal of Peking University (Health Sciences) ›› 2022, Vol. 54 ›› Issue (4): 663-668. doi: 10.19723/j.issn.1671-167X.2022.04.013
Previous Articles Next Articles
Establishment of a mutation prediction model for evaluating the efficacy of immunotherapy in renal carcinoma
Cai-peng QIN,Yu-xuan SONG,Meng-ting DING,Fei WANG,Jia-xing LIN,Wen-bo YANG,Yi-qing DU,Qing LI,Shi-jun LIU,Tao XU*( )
)
- Department of Urology, Peking University People' s Hospital, Beijing 100044, China
CLC Number:
- R737
| 1 |
Sung H , Ferlay J , Siegel RL , et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71 (3): 209- 249.
doi: 10.3322/caac.21660 |
| 2 |
Moch H , Cubilla AL , Humphrey PA , et al. The 2016 WHO classification of tumours of the urinary system and male genital organs: Part A: Renal, penile, and testicular tumours[J]. Eur Urol, 2016, 70 (1): 93- 105.
doi: 10.1016/j.eururo.2016.02.029 |
| 3 |
Ht C , McGovern F . Renal-cell carcinoma[J]. N Engl J Med, 2005, 353 (23): 2477- 2490.
doi: 10.1056/NEJMra043172 |
| 4 |
Bianchi M , Sun M , Jeldres C , et al. Distribution of metastatic sites in renal cell carcinoma: A population-based analysis[J]. Ann Oncol, 2012, 23 (4): 973- 980.
doi: 10.1093/annonc/mdr362 |
| 5 |
Everson TC , Cole WH . Spontaneous regression of cancer: Preliminary report[J]. Ann Surg, 1956, 144 (3): 366- 383.
doi: 10.1097/00000658-195609000-00007 |
| 6 | Janiszewska AD , Poletajew S , Wasiutyński A . Spontaneous regression of renal cell carcinoma[J]. Contemp Oncol (Pozn), 2013, 17 (2): 123- 127. |
| 7 |
Klapper JA , Downey SG , Smith FO , et al. High-dose interleukin-2 for the treatment of metastatic renal cell carcinoma: A retrospective analysis of response and survival in patients treated in the surgery branch at the National Cancer Institute between 1986 and 2006[J]. Cancer, 2008, 113 (2): 293- 301.
doi: 10.1002/cncr.23552 |
| 8 |
Motzer RJ , Escudier B , McDermott DF , et al. Nivolumab versus everolimus in advanced renal-cell carcinoma[J]. N Engl J Med, 2015, 373 (19): 1803- 1813.
doi: 10.1056/NEJMoa1510665 |
| 9 |
Motzer RJ , Tannir NM , McDermott DF , et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma[J]. N Engl J Med, 2018, 378 (14): 1277- 1290.
doi: 10.1056/NEJMoa1712126 |
| 10 | Plimack ER , Rini BI , Stus V , et al. Pembrolizumab plus axitinib versus sunitinib as first-line therapy for advanced renal cell carcinoma (RCC): Updated analysis of KEYNOTE-426[J]. J Clin Oncol, 2020, 38 (Suppl 15): 5001. |
| 11 |
Samstein RM , Lee CH , Shoushtari AN , et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types[J]. Nat Genet, 2019, 51 (2): 202- 206.
doi: 10.1038/s41588-018-0312-8 |
| 12 |
Braun DA , Hou Y , Bakouny Z , et al. Interplay of somatic alterations and immune infiltration modulates response to PD-1 blockade in advanced clear cell renal cell carcinoma[J]. Nat Med, 2020, 26 (6): 909- 918.
doi: 10.1038/s41591-020-0839-y |
| 13 |
McDermott DF , Huseni MA , Atkins MB , et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma[J]. Nat Med, 2018, 24 (6): 749- 757.
doi: 10.1038/s41591-018-0053-3 |
| 14 |
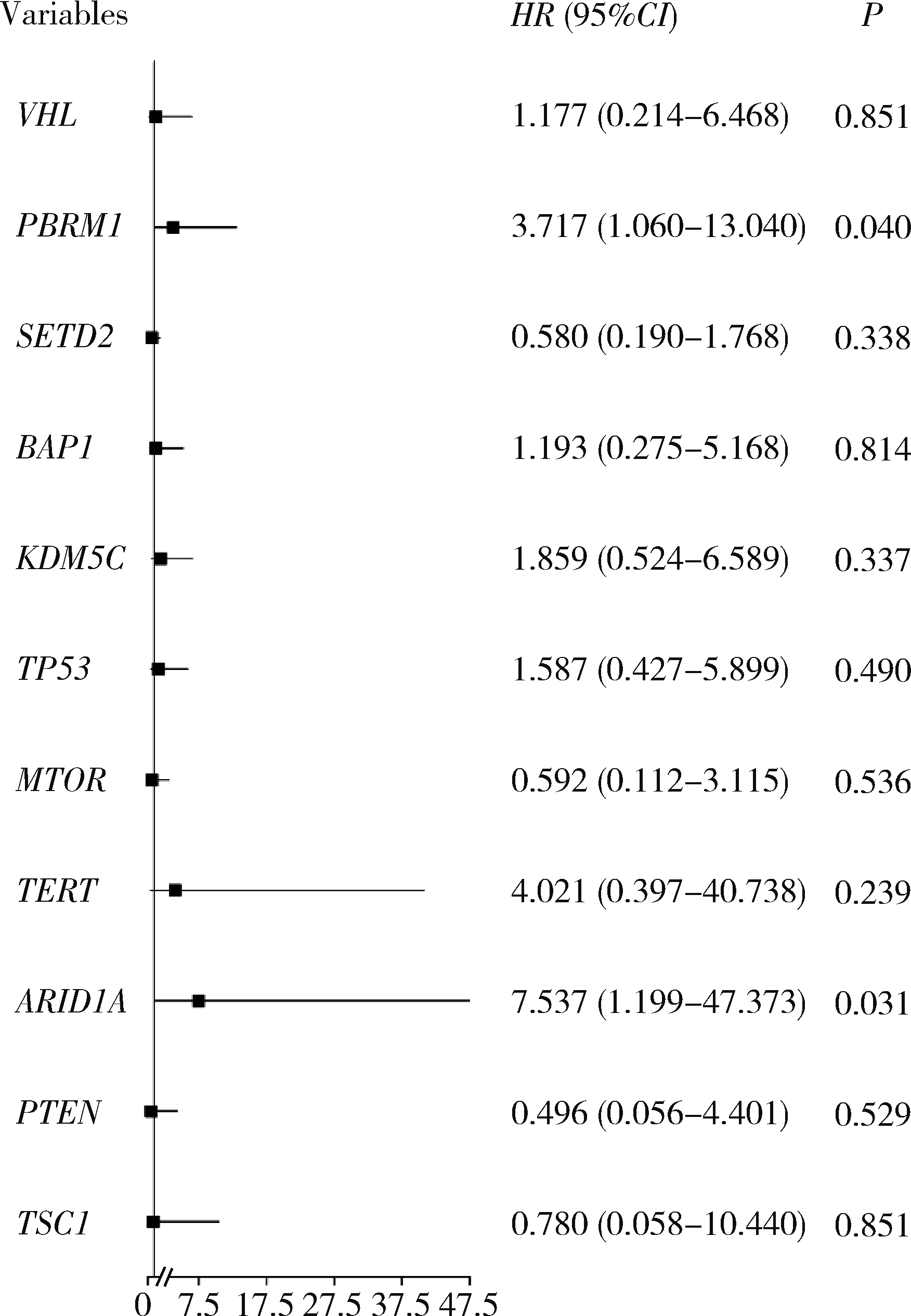
Braun DA , Ishii Y , Walsh AM , et al. Clinical validation of PBRM1 alterations as a marker of immune checkpoint inhibitor response in renal cell carcinoma[J]. JAMA Oncol, 2019, 5 (11): 1631- 1633.
doi: 10.1001/jamaoncol.2019.3158 |
| 15 |
Motzer RJ , Banchereau R , Hamidi H , et al. Molecular subsets in renal cancer determine outcome to checkpoint and angiogenesis blockade[J]. Cancer cell, 2020, 38 (6): 803- 817.
doi: 10.1016/j.ccell.2020.10.011 |
| 16 |
Hwang J , Kim H , Han J , et al. Identification of survival-specific genes in clear cell renal cell carcinoma using a customized next-generation sequencing gene panel[J]. J Pers Med, 2022, 12 (1): 113.
doi: 10.3390/jpm12010113 |
| 17 |
Bui TO , Feugeas JP , Pamoukdjian F , et al. Genomics of clear-cell renal cell carcinoma: A systematic review and meta-analysis[J]. Eur Urol, 2022, 81 (4): 349- 361.
doi: 10.1016/j.eururo.2021.12.010 |
| 18 | Zhou C , Niu Y , Ma T , et al. The predictive values of ARID1A mutations for response to immune checkpoint inhibitors are varied in different types of solid tumors[J]. Cancer Res, 2021, 81 (Suppl 13): 1641. |
| 19 |
De Velasco G , Miao D , Voss MH , et al. Tumor mutational load and immune parameters across metastatic renal cell carcinoma risk groups[J]. Cancer Immunol Res, 2016, 4 (10): 820- 822.
doi: 10.1158/2326-6066.CIR-16-0110 |
| 20 |
Chen DS , Mellman I . Elements of cancer immunity and the cancer-immune set point[J]. Nature, 2017, 541 (7637): 321- 330.
doi: 10.1038/nature21349 |
| 21 |
Binnewies M , Roberts EW , Kersten K , et al. Understanding the tumor immune microenvironment (TIME) for effective therapy[J]. Nat Med, 2018, 24 (5): 541- 550.
doi: 10.1038/s41591-018-0014-x |
| [1] | Fan SHU,Yichang HAO,Zhanyi ZHANG,Shaohui DENG,Hongxian ZHANG,Lei LIU,Guoliang WANG,Xiaojun TIAN,Lei ZHAO,Lulin MA,Shudong ZHANG. Functional and oncologic outcomes of partial nephrectomy for cystic renal cell carcinoma: A single-center retrospective study [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 667-672. |
| [2] | Zezhen ZHOU,Shaohui DENG,Ye YAN,Fan ZHANG,Yichang HAO,Liyuan GE,Hongxian ZHANG,Guoliang WANG,Shudong ZHANG. Predicting the 3-year tumor-specific survival in patients with T3a non-metastatic renal cell carcinoma [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 673-679. |
| [3] | Yun-chong LIU,Zong-long WU,Li-yuan GE,Tan DU,Ya-qian WU,Yi-meng SONG,Cheng LIU,Lu-lin MA. Mechanism of nuclear protein 1 in the resistance to axitinib in clear cell renal cell carcinoma [J]. Journal of Peking University (Health Sciences), 2023, 55(5): 781-792. |
| [4] | Dong LAN,Zhuo LIU,Yu-xuan LI,Guo-liang WANG,Xiao-jun TIAN,Lu-lin MA,Shu-dong ZHANG,Hong-xian ZHANG. Risk factors for massive hemorrhage after radical nephrectomy and removal of venous tumor thrombus [J]. Journal of Peking University (Health Sciences), 2023, 55(5): 825-832. |
| [5] | Yun-fei SHI,Hao-jie WANG,Wei-ping LIU,Lan MI,Meng-ping LONG,Yan-fei LIU,Yu-mei LAI,Li-xin ZHOU,Xin-ting DIAO,Xiang-hong LI. Analysis of clinicopathological and molecular abnormalities of angioimmunoblastic T-cell lymphoma [J]. Journal of Peking University (Health Sciences), 2023, 55(3): 521-529. |
| [6] | Yun-yi XU,Zheng-zheng SU,Lin-mao ZHENG,Meng-ni ZHANG,Jun-ya TAN,Ya-lan YANG,Meng-xin ZHANG,Miao XU,Ni CHEN,Xue-qin CHEN,Qiao ZHOU. Read-through circular RNA rt-circ-HS promotes hypoxia inducible factor 1α expression and renal carcinoma cell proliferation, migration and invasiveness [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 217-227. |
| [7] | Qi SHEN,Yi-xiao LIU,Qun HE. Mucinous tubular and spindle cell carcinoma of kidney: Clinicopathology and prognosis [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 276-282. |
| [8] | Quan ZHANG,Hai-feng SONG,Bing-lei MA,Zhe-nan ZHANG,Chao-hui ZHOU,Ao-lin LI,Jun LIU,Lei LIANG,Shi-yu ZHU,Qian ZHANG. Pre-operative prognostic nutritional index as a predictive factor for prognosis in non-metastatic renal cell carcinoma treated with surgery [J]. Journal of Peking University (Health Sciences), 2023, 55(1): 149-155. |
| [9] | Er-shu BO,Peng HONG,Yu ZHANG,Shao-hui DENG,Li-yuan GE,Min LU,Nan LI,Lu-lin MA,Shu-dong ZHANG. Clinicopathological features and prognostic analysis of papillary renal cell carcinoma [J]. Journal of Peking University (Health Sciences), 2022, 54(4): 615-620. |
| [10] | Tian-yu CAI,Zhen-peng ZHU,Chun-ru XU,Xing JI,Tong-de LV,Zhen-ke GUO,Jian LIN. Expression and significance of fibroblast growth factor receptor 2 in clear cell renal cell carcinoma [J]. Journal of Peking University (Health Sciences), 2022, 54(4): 628-635. |
| [11] | Mei-ni ZUO,Yi-qing DU,Lu-ping YU,Xiang DAI,Tao XU. Correlation between metabolic syndrome and prognosis of patients with clear cell renal cell carcinoma [J]. Journal of Peking University (Health Sciences), 2022, 54(4): 636-643. |
| [12] | Yi-cen YING,Qi TANG,Kai-wei YANG,Yue MI,Yu FAN,Wei YU,Yi SONG,Zhi-song HE,Li-qun ZHOU,Xue-song LI. Clinical features of immune checkpoint inhibitor-related myositis in patients with urological cancer [J]. Journal of Peking University (Health Sciences), 2022, 54(4): 644-651. |
| [13] | Sheng-jie LIU,Hui-min HOU,Zheng-tong LV,Xin DING,Lu WANG,Lei ZHANG,Ming LIU. Bipolar androgen therapy followed by immune checkpoint inhibitors in metastatic castration resistant prostate cancer: A report of 4 cases [J]. Journal of Peking University (Health Sciences), 2022, 54(4): 766-769. |
| [14] | GU Yang-chun,LIU Ying,XIE Chao,CAO Bao-shan. Pituitary immune-related adverse events induced by programmed cell death protein 1 inhibitors in advanced lung cancer patients: A report of 3 cases [J]. Journal of Peking University (Health Sciences), 2022, 54(2): 369-375. |
| [15] | Yu TIAN,Xiao-yue CHENG,Hui-ying HE,Guo-liang WANG,Lu-lin MA. Clinical and pathological features of renal cell carcinoma with urinary tract tumor thrombus: 6 cases report and literature review [J]. Journal of Peking University (Health Sciences), 2021, 53(5): 928-932. |
|
||