北京大学学报(医学版) ›› 2019, Vol. 51 ›› Issue (2): 197-205. doi: 10.19723/j.issn.1671-167X.2019.02.001
• 论著 • 下一篇
小鼠卡英酸颞叶癫痫慢性发作期的磷酸化蛋白组学研究
孙智明1,陈倩1,李明华1,马维宁2,赵旭阳1,3,∆( ),黄卓1,∆(
),黄卓1,∆( )
)
- 1. 北京大学药学院分子与细胞药理学系 天然药物与仿生药物国家重点实验室 系统生物医学研究所, 北京 100191
2. 中国医科大学附属盛京医院神经外科, 沈阳 110000
3. 北京大学基础医学院北京肿瘤系统生物学重点实验室, 北京 100191
Chronic phosphoproteomic in temporal lobe epilepsy mouse models induced by kainic acid
Zhi-ming SUN1,Qian CHEN1,Ming-hua LI1,Wei-ning MA2,Xu-yang ZHAO1,3,∆( ),Zhuo HUANG1,∆(
),Zhuo HUANG1,∆( )
)
- 1. Institute of Systems Biomedicine, State Key Laboratory of Natural and Biomimetic Drugs, Department of Molecular and Cellular Pharmacology, Peking University School of Pharmaceutical Science, Beijing 100191, China
2. Department of Neurosurgery, Sheng Jing Hospital affiliated to China Medical University, Shenyang 110000, China
3. Beijing Key Laboratory of Tumor Systems Biology, Peking University School of Basic Medical Science, Beijing 100191, China
摘要:
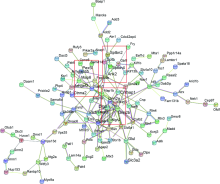
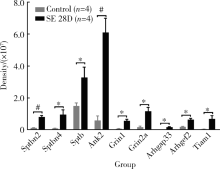
目的: 探究小鼠颞叶癫痫慢性发作期蛋白质功能和信号通路的改变。方法: (1)制备小鼠卡英酸颞叶癫痫模型,行为学达到Racine分级4分判定为造模成功。28 d后,取对照组和实验组小鼠海马组织进行磷酸化蛋白组学实验;(2) 选取检出密度大于10 6的数据进行统计分析;(3)利用GO(Gene Ontology)数据库、KEGG(Kyoto Encyclopedia of Genes and Genomes)数据库和STRING数据库对磷酸化蛋白组学数据进行统计分析;(4)结合文献对组学结果进行分析。 结果: (1)质谱共检测出12 697个蛋白质磷酸化位点,其中159个位点变化差异具有统计学意义(P<0.05);(2)在蛋白质功能层面,磷酸化水平显著性变化的蛋白质的分子功能主要是结合(39.5%)和催化活性(35.7%), 这些蛋白质参与细胞交流(20.8%)、初级代谢和含磷酸盐化合物代谢等生化过程;(3)在信号通路层面,这些蛋白质参与10条信号转导通路,包括谷氨酸能突触信号通路、Ras信号通路、长时程增强信号通路等;(4)在蛋白质相互作用层面,这些蛋白质形成以Grin1和Dlg3为核心,以Arhgef 2、Arhgap33和Tiam1为核心与以Spnb1/3/4、Add3和Ank2为核心的蛋白质相互作用网;(5)磷酸化蛋白组学数据显示,Grin1、Arhgef2、Arhgap33、Tiam1、Sptbn1/2/4和Ank2等磷酸化水平在癫痫慢性发作期显著升高。结论: 磷酸化蛋白组学的结果从蛋白质功能、信号通路和蛋白质相互作用3个层面阐明了小鼠颞叶癫痫慢性发作期海马体蛋白质的变化,验证了磷酸化蛋白组学研究的可靠性,并提示多巴胺功能和Kir3.1钾通道功能可能与癫痫发生相关。
中图分类号:
- R393
| [1] | 郭铭花, 张敬军 . 癫痫流行病学调查研究[J]. 中华脑科疾病与康复杂志: 电子版, 2013,3(5):46-48. |
| [2] |
Hesdorffer DC, Tomson T . Sudden unexpected death in epilepsy. Potential role of antiepileptic drugs[J]. CNS Drugs, 2013,27(2):113-119.
doi: 10.1007/s40263-012-0006-1 |
| [3] | 佟晓燕, 王玉平 . 成年癫痫患者抑郁、焦虑状况及生活质量调查[J]. 脑与神经疾病杂志, 2009,17(2):123-126. |
| [4] |
Brodie MJ, Kwan P . Newer drugs for focal epilepsy in adults[J]. BMJ, 2012,344:e345.
doi: 10.1136/bmj.e345 |
| [5] |
Schmidt D, Sillanpää M . Evidence-based review on the natural history of the epilepsies[J]. Curr Opin Neurol, 2012,25(2):159.
doi: 10.1097/WCO.0b013e3283507e73 |
| [6] |
Palleria C, Coppola A, Citraro R , et al. Perspectives on treatment options for mesial temporal lobe epilepsy with hippocampal sclerosis[J]. Expert Opin Pharmacother, 2015,16(15):2355.
doi: 10.1517/14656566.2015.1084504 |
| [7] |
Williams AD, Jung S, Poolos NP . Protein kinase C bidirectionally modulates Ih and hyperpolarization-ctivated cyclic nucleotide-ated (HCN) channel surface expression in hippocampal pyramidal neurons[J]. J Physiol, 2015,593(13):2779-2792.
doi: 10.1113/JP270453 |
| [8] |
Takeichi M . Stability of dendritic spines and synaptic contacts is controlled by aN-catenin[J]. Nat Neurosci, 2004,7(4):357-363.
doi: 10.1038/nn1212 |
| [9] |
Togashi H, Abe K, Mizoguchi A , et al. Cadherin regulates dendritic spine morphogenesis[J]. Neuron, 2002,35(1):77-89.
doi: 10.1016/S0896-6273(02)00748-1 |
| [10] |
Park C, Falls W, Finger JH , et al. Deletion in Catna2, encoding alpha N-catenin, causes cerebellar and hippocampal lamination defects and impaired startle modulation[J]. Nat Genet, 2002,31(3):279-284.
doi: 10.1038/ng908 |
| [11] |
Huang C, Fu XH, Zhou D , et al. The role of Wnt/β-catenin signaling pathway in disrupted hippocampal neurogenesis of temporal lobe epilepsy: a potential therapeutic target[J]. Neurochem Res, 2015,40(7):1319.
doi: 10.1007/s11064-015-1614-1 |
| [12] | Tóth K, Maglóczky Z . The vulnerability of calretinin-containing hippocampal interneurons to temporal lobe epilepsy[J]. Front Neuroanat, 2014,8:100. |
| [13] |
Hardies K, Cai Y, Jardel C , et al. Loss of SYNJ1 dual phosphatase activity leads to early onset refractory seizures and progressive neurological decline[J]. Brain, 2016,139(9):2420-2430.
doi: 10.1093/brain/aww180 |
| [14] |
Milosevic I, Giovedi S, Lou X , et al. Recruitment of endophilin to clathrin coated pit necks is required for efficient vesicle uncoating after fission[J]. Neuron, 2011,72(4):587-601.
doi: 10.1016/j.neuron.2011.08.029 |
| [15] |
Di PG, Sankaranarayanan S, Wenk MR , et al. Decreased synaptic vesicle recycling efficiency and cognitive deficits in amphiphysin 1 knockout mice[J]. Neuron, 2002,33(5):789-804.
doi: 10.1016/S0896-6273(02)00601-3 |
| [16] |
Eid T, Tu N, Lee TS , et al. Regulation of astrocyte glutamine synthetase in epilepsy[J]. Neurochem Int, 2013,63(7):670-681.
doi: 10.1016/j.neuint.2013.06.008 |
| [17] |
Cerfontain H, Telder MA, Vollbracht L . Inborn error of amino acid synjournal: human glutamine synthetase deficiency[J]. J Inherit Metab Dis, 2006,29(2/3):352.
doi: 10.1007/s10545-006-0256-5 |
| [18] |
Upreti C, Otero R, Partida C , et al. Altered neurotransmitter release, vesicle recycling and presynaptic structure in the pilocarpine model of temporal lobe epilepsy[J]. Brain, 2012,135(Pt 3):869-885.
doi: 10.1093/brain/awr341 |
| [19] |
Putkonen N, Kukkonen JP, Mudo G , et al. Involvement of cyclin-dependent kinase-5 in the kainic acid-mediated degeneration of glutamatergic synapses in the rat hippocampus[J]. Eur J Neurosci, 2011,34(8):1212-1221.
doi: 10.1111/j.1460-9568.2011.07858.x |
| [20] | Dingledine R . Glutamatergic mechanisms related to epilepsy: ionotropic receptors[J]. 2010,51(s5):15. |
| [21] |
Kulik A . Compartment-dependent colocalization of Kir3.2-con-taining K+ channels and GABAB receptors in hippocampal pyramidal cells[J]. J Neurosci, 2006,26(16):4289.
doi: 10.1523/JNEUROSCI.4178-05.2006 |
| [22] | Tonini R, Franceschetti S, Parolaro D , et al. Involvement of CDC25Mm/Ras-GRF1-dependent signaling in the control of neuronal excitability[J]. Mol Cell Neurosci, 2002,18(6):691-701. |
| [23] |
Zhu Q, Wang L, Xiao Z , et al. Decreased expression of Ras-GRF1 in the brain tissue of the intractable epilepsy patients and experimental rats[J]. Brain Res, 2013,1493(1):99-109.
doi: 10.1016/j.brainres.2012.11.033 |
| [24] |
Moschovos C, Kostopoulos G, Papatheodoropoulos C . Long-term potentiation of high-frequency oscillation and synaptic transmission characterize in vitro NMDA receptor-dependent epileptogenesis in the hippocampus[J]. Neurobiol Dis, 2008,29(2):368.
doi: 10.1016/j.nbd.2007.09.007 |
| [25] | Lenz M, Ben SM, Deller T , et al. Pilocarpine-induced status epilepticus is associated with changes in the actin-modulating protein synaptopodin and alterations in long-term potentiation in the mouse hippocampus[J]. Neural Plast, 2017,2017:2652560. |
| [26] |
Liu JX, Hu M, Chen XL , et al. Reducedexpression of phospholipase C beta in hippocampal interneuron during pilocarpine induced status epilepticus in mice[J]. Neurochem Int, 2014,68(1):10.
doi: 10.1016/j.neuint.2014.01.009 |
| [27] |
Kurian MA, Meyer E, Vassallo G , et al. Phospholipase C beta 1 deficiency is associated with early-onset epileptic encephalopathy[J]. Brain, 2010,133(10):2964-2970.
doi: 10.1093/brain/awq238 |
| [28] |
Chen W, Yuan H . GRIN1 mutations in early-onset epileptic encephalopathy[J]. Pediatr Neurol Briefs, 2015,29(6):44.
doi: 10.15844/pedneurbriefs-29-6 |
| [29] |
Mikuni N, Babb TL, Chakravarty DN , et al. NMDAR2 upregulation precedes mossy fiber sprouting in kainate rat hippocampal epilepsy[J]. Neurosci Lett, 1998,255(1):25.
doi: 10.1016/S0304-3940(98)00704-6 |
| [30] |
Buckmaster PS . Does mossy fiber sprouting give rise to the epileptic state[J]. Adv Exp Med Biol, 2014,813:161-168.
doi: 10.1007/978-94-017-8914-1 |
| [31] |
Brouns MR, Matheson SF , Settleman J. p190 RhoGAP is the principal Src substrate in brain and regulates axon outgrowth, guidance and fasciculation[J]. Nat Cell Biol, 2001,3(4):361-367.
doi: 10.1038/35070042 |
| [32] | Stankiewicz TR, Linseman DA . Rho family GTPases: key players in neuronal development, neuronal survival, and neurodegeneration[J]. Front Cell Neurosci, 2014,8:314. |
| [33] |
Rocha L, Alonso-Vanegas M, Villeda-Hernandez J , et al. Dopamine abnormalities in the neocortex of patients with temporal lobe epilepsy[J]. Neurobiol Dis, 2012,45(1):499-507.
doi: 10.1016/j.nbd.2011.09.006 |
| [34] |
Kaupmann K, Schuler V, Mosbacher J , et al. Human γ-aminobutyric acid type B receptors are differentially expressed and regulate inwardly rectifying K+ channels[J]. Proc Natl Acad Sci USA, 1998,95(25):14991-14996.
doi: 10.1073/pnas.95.25.14991 |
| [1] | 朱莎,徐宗胜,夏晴,方筱静,赵丹华,刘献增. 伴杏仁核肥大的颞叶癫痫的临床及病理特征[J]. 北京大学学报(医学版), 2019, 51(5): 824-828. |
|
||