北京大学学报(医学版) ›› 2019, Vol. 51 ›› Issue (3): 536-541. doi: 10.19723/j.issn.1671-167X.2019.03.024
抗缪勒管激素用于戈舍瑞林在年轻乳腺癌患者化疗期间保护卵巢储备功能的评价
- 北京大学人民医院乳腺外科, 北京 100044
Anti-Müllerian hormone as a new marker of the ovarian reserve function preservation by goserelin during (neo)adjuvant chemotherapy for young breast cancer patients
- Department of Breast Surgery, Peking University People’s Hospital, Beijing 100044, China
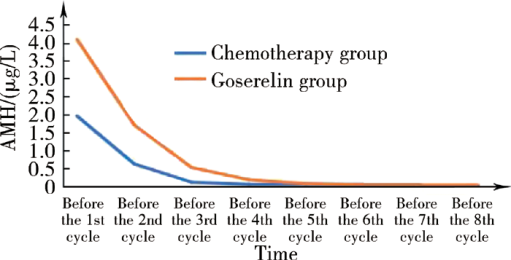
摘要: 目的 监测反映卵巢储备功能的最佳生化指标——抗缪勒管激素(anti-Müllerian hormone,AMH)在化疗前和化疗结束后1年内的动态变化,评价促性腺激素释放激素激动剂(gonadotropin-releasing hormone agonist, GnRHa)戈舍瑞林在年轻乳腺癌患者化疗期间保护卵巢储备功能的效果。方法 选择2015年12月至2017年6月在北京大学人民医院乳腺外科就诊的年龄≤45岁Ⅰ~Ⅲ期乳腺癌患者101位,根据患者意愿,无干预分至化疗联合戈舍瑞林组(戈舍瑞林组)和单纯化疗组(化疗组)。在化疗开始前、化疗期间、化疗结束后半年、化疗结束后1年,连续监测两组患者的血清AMH和月经状态。首要研究终点是化疗结束后1年AMH低水平(<0.4 μg /L),次要研究终点是闭经(入组后停经时间超过12个月)。结果 51位患者选择单纯化疗,50位患者选择化疗联合戈舍瑞林保护卵巢功能。临床病理基线资料显示,未婚未育、生育意愿强烈、成功保乳、激素受体阴性、处于疾病Ⅰ期或Ⅱ期的乳腺癌患者更多地在化疗前选用戈舍瑞林保护卵巢功能。化疗结束后1年,化疗组患者AMH低水平率显著高于戈舍瑞林组患者 (74.5% vs. 38.0%, P<0.001), 闭经率也与AMH低水平率相一致(56.9% vs.24.0%, P=0.001),并且戈舍瑞林组的患者更多在化疗结束后6个月内恢复月经(78.9% vs.54.5%), AMH升至0.4 μg /L以上(71.0% vs.53.8%)。亚组分析中,无论年龄分组、化疗方案分组或化疗后是否口服他莫昔芬分组,戈舍瑞林组患者的血清AMH值和月经均恢复得更多。在化疗结束后1年,化疗组月经恢复的22人中有8人(36.4%), 戈舍瑞林组月经恢复的38人中有7人(18.4%)AMH仍处于低水平。此外,对化疗组20位患者和戈舍瑞林组21位患者在化疗过程中动态监测AMH和月经状态,化疗组患者的AMH均值在化疗开始后快速下降,在第3周期化疗前降至极低水平,此时70%的患者还未停经;戈舍瑞林组患者在第3周期化疗前全部停经,但AMH均值未降至低水平。结论 由于选择联合治疗的患者其血清AMH值在化疗结束后更多、更早地恢复,故对戈舍瑞林保护年轻乳腺癌患者卵巢储备功能的有效性提供了证据。相比月经状态,AMH更能精准地用于临床评价化疗前后年轻乳腺癌患者的卵巢储备功能。
中图分类号:
- R737.9
| [1] |
Smigal C, Jemal A, Ward E , et al. Trends in breast cancer by race and ethnicity: update 2006[J]. CA Cancer J Clin, 2006,56(3):168-183.
doi: 10.3322/canjclin.56.3.168 |
| [2] |
Ganz PA, Hahn EE . Implementing a survivorship care plan for patients with breast cancer[J]. J Clin Oncol, 2008,26(5):759-767.
doi: 10.1200/JCO.2007.14.2851 |
| [3] |
Warne GL, Fairley KF, Hobbs JB , et al. Cyclophosphamide-induced ovarian failure[J]. N Engl J Med, 1973,289(22):1159-1162.
doi: 10.1056/NEJM197311292892202 |
| [4] |
Anderson RA, Themmen AP, Al-Qahtani A , et al. The effects of chemotherapy and long-term gonadotrophin suppression on the ovarian reserve in premenopausal women with breast cancer[J]. Hum Reprod, 2006,21(10):2583-2592.
doi: 10.1093/humrep/del201 |
| [5] |
Partridge AH, Gelber S, Peppercorn J , et al. Fertility and menopausal outcomes in young breast cancer survivors[J]. Clin Breast Cancer, 2008,8(1):65-69.
doi: 10.3816/CBC.2008.n.004 |
| [6] |
Schover LR . Premature ovarian failure and its consequences: vasomotor symptoms, sexuality, and fertility[J]. J Clin Oncol, 2008,26(5):753-758.
doi: 10.1200/JCO.2007.14.1655 |
| [7] |
Del Mastro L, Catzeddu T, Boni L , et al. Prevention of chemotherapy-induced meno-pause by temporary ovarian suppression with goserelin in young, early breast cancer patients[J]. Ann Oncol, 2006,17(1):74-78.
doi: 10.1093/annonc/mdj029 |
| [8] |
Moore HC, Unger JM, Phillips KA , et al. Goserelin for ovarian protection during breast-cancer adjuvant chemotherapy[J]. N Engl J Med, 2015,372(10):923-932.
doi: 10.1056/NEJMoa1413204 |
| [9] | Lambertini M, Ceppi M, Poggio F , et al. Ovarian suppression using luteinizing hormone releasing hormone agonists during chemotherapy to preserve ovarian function and fertility of breast cancer patients: a meta-analysis of randomized studies[J]. Ann Oncol, 2015,26(12):2408-2419. |
| [10] |
Lambertini M, Moore H, Leonard R , et al. Gonadotropin-releasing hormone agonists during chemotherapy for preservation of ovarian function and fertility in premenopausal patients with early breast cancer: a systematic review and meta-analysis of individual patient-level data[J]. J Clin Oncol, 2018,36(19):1981-1990.
doi: 10.1200/JCO.2018.78.0858 |
| [11] | National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Breast Cancer, Version 2. 2013 [S/OL]. ( 2013-03-11)[2019-03-01].https://www.nccn.org/professionals/physician_gls/f_guidelines.asp. |
| [12] |
Sun W, Stegmann BJ, Henne M , et al. A new approach to ovarian reserve testing[J]. Fertil Steril, 2008,90(6):2196-2202.
doi: 10.1016/j.fertnstert.2007.10.080 |
| [13] |
La Marca A, Broekmans FJ, Volpe A , et al. Anti-Müllerian hormone (AMH): what do we still need to know[J]. Hum Reprod, 2009,24(9):2264-2275.
doi: 10.1093/humrep/dep210 |
| [14] |
Himabindu Y, Sriharibabu M, Gopinathan K , et al. Anti-Müllerian hormone and antral follicle count as predictors of ovarian response in assisted reproduction[J]. J Hum Reprod Sci, 2013,6(1):27-31.
doi: 10.4103/0974-1208.112377 |
| [15] | Del Mastro L, Boni L, Michelotti A , et al. Effect of the gonadotropin-releasing hormone analogue triptorelin on the occurrence of chemotherapy-induced early menopause in premenopausal women with breast cancer[J]. JAMA, 2011,306(3):269-276. |
| [16] |
Gerber B, von Minckwitz G, Stehle H , et al. Effect of luteinizing hormone-releasing hormone agonist on ovarian function after modern adjuvant breast cancer chemotherapy: the GBG 37 ZORO study[J]. J Clin Oncol, 2011,29(17):2334-2341.
doi: 10.1200/JCO.2010.32.5704 |
| [17] |
Munster PN, Moore AP, Ismailkhan R , et al. Randomized trial using gonadotropin-releasing hormone agonist triptorelin for the preservation of ovarian function during (neo) adjuvant chemotherapy for breast cancer[J]. J Clin Oncol, 2012,30(5):533-538.
doi: 10.1200/JCO.2011.34.6890 |
| [1] | 张云静,乔丽颖,祁萌,严颖,亢伟伟,刘国臻,王明远,席云峰,王胜锋. 乳腺癌患者新发心血管疾病预测模型的建立与验证:基于内蒙古区域医疗数据[J]. 北京大学学报(医学版), 2023, 55(3): 471-479. |
| [2] | 朱晓娟,张虹,张爽,李东,李鑫,徐玲,李挺. 人表皮生长因子受体2低表达乳腺癌的临床病理学特征及预后[J]. 北京大学学报(医学版), 2023, 55(2): 243-253. |
| [3] | 王跃,张爽,张虹,梁丽,徐玲,程元甲,段学宁,刘荫华,李挺. 激素受体阳性/人表皮生长因子受体2阴性乳腺癌临床病理特征及预后[J]. 北京大学学报(医学版), 2022, 54(5): 853-862. |
| [4] | 宋国红,李惠平,邸立军,严颖,姜晗昉,徐玲,万冬桂,李瑛,王墨培,肖宇,张如艳,冉然,王环. 真实世界吡咯替尼治疗HER2阳性转移性乳腺癌的疗效及安全性[J]. 北京大学学报(医学版), 2020, 52(2): 254-260. |
| [5] | 李秀楠,刘爱蕙,唐欣,任宇. 尿路上皮癌相关1基因通过竞争性抑制miR-18a增强乳腺癌细胞的他莫昔芬治疗耐药[J]. 北京大学学报(医学版), 2017, 49(2): 295-302. |
| [6] | 邵彬,李惠平,邸立军,宋国红,姜晗昉,梁旭,王超颖,严颖,林晓琳,王丽娜,宛凤玲,. 外周血淋巴细胞亚群监测对复发转移性乳腺癌的预测及预后价值[J]. 北京大学学报(医学版), 2016, 48(2): 304-309. |
|
||