北京大学学报(医学版) ›› 2019, Vol. 51 ›› Issue (3): 542-547. doi: 10.19723/j.issn.1671-167X.2019.03.025
利用转录组二代测序探索直肠癌术前放化疗的敏感性分子特征
Detection of preoperative chemoradiotherapy sensitivity molecular characteristics of rectal cancer by transcriptome second generation sequencing
Wei ZHANG1,Ying-jiang YE2,Xian-wen REN3,Jing HUANG4,Zhan-long SHEN1△( )
)
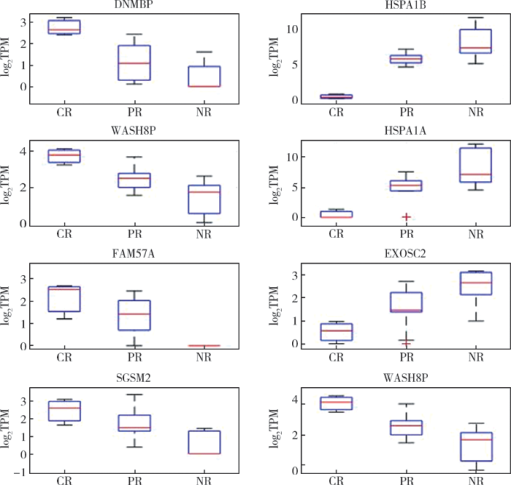
摘要: 目的 探索直肠癌新辅助放化疗的敏感性分子特征。方法 前瞻性收集局部进展期中、低位直肠癌30例患者的临床病理资料,包括一般情况、放化疗前影像学资料、放化疗前活组织病理检查资料、肿瘤分化程度等9项指标,分析其与直肠癌放化疗后肿瘤消退分级(tumor regression grading,TRG)的相关性。收集这30例患者新辅助治疗前结肠镜活检冰冻标本,进行转录组二代测序和生物信息学分析,筛选可能驱动直肠癌放化疗敏感性的基因。结果 30例直肠癌患者中,病理完全缓解9例,部分缓解12例,缓解差9例。直肠癌放化疗后病理TRG缓解程度与肿瘤术前MRI的T分期呈负相关(P=0.046),与术前MRI直肠癌壁外血管侵犯(extramural vascular invasion, EMVI)呈正相关(P=0.003)。转录组二代测序对所获取的P<0.05的217条转录本进行信号通路富集分析,可以发现多条与抗原呈递相关的细胞信号转导通路,其中HSPA1A、HSPA1B和EXOSC2的高表达和术后病理缓解差呈正相关(P<0.05),DNMBP、WASH8P、FAM57A和SGSM2等的高表达和术后病理缓解良好呈正相关(P<0.05)。结论 直肠癌术前MRI检测肿瘤EMVI阳性的患者放化疗后病理完全缓解率明显优于EMVI阴性者。HSPA1A、HSPA1B和EXOSC2高表达的患者术后病理缓解差,而DNMBP、WASH8P、FAM57A和SGSM2高表达的患者术后病理缓解良好。基于直肠癌放化疗敏感性分子特征,尝试阻断或增强与直肠癌放化疗敏感性相关的分子通路,可进一步探索能增加直肠癌放化疗敏感性的候选治疗靶点。
中图分类号:
- R735.3
| [1] |
Park IJ, You YN, Agarwal A , et al. Neoadjuvant treatment response as an early response indicator for patients with rectal cancer[J]. J Clin Oncol, 2012,30(15):1770-1776.
doi: 10.1200/JCO.2011.39.7901 |
| [2] |
Dworak O, Keilholz L, Hoffmann A . Pathological features of rectal cancer after preoperative radiochemotherapy[J]. Int J Colorectal Dis, 1997,12(1):19-23.
doi: 10.1007/s003840050072 |
| [3] |
Rödel C, Martus P, Papadoupolos T , et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer[J]. J Clin Oncol, 2005,23(34):8688-8696.
doi: 10.1200/JCO.2005.02.1329 |
| [4] |
Fokas E, Liersch T, Fietkau R , et al. Tumor regression grading after preoperative chemoradiotherapy for locally advanced rectal carcinoma revisited: updated results of the CAO/ARO/AIO-94 trial[J]. J Clin Oncol, 2014,32(15):1554-1562.
doi: 10.1200/JCO.2013.54.3769 |
| [5] |
Smith JD, Ruby JA, Goodman KA , et al. Nonoperative management of rectal cancer with complete clinical response after neoadjuvant therapy[J]. Ann Surg, 2012,256(6):965-972.
doi: 10.1097/SLA.0b013e3182759f1c |
| [6] |
Postmus I, Trompet S, Deshmukh HA , et al. Pharmacogenetic meta-analysis of genome-wide association studies of LDL choleste-rol response to statins[J]. Nat Commun, 2014,5:5068.
doi: 10.1038/ncomms6068 |
| [7] | Williamson JS, Jones HG, Williams N , et al. Extramural vascular invasion and response to neoadjuvant chemoradiotherapy in rectal cancer: influence of the CpG island methylator phenotype[J]. World J Gastrointest Oncol, 2017,9(5):209-217. |
| [8] |
Chand M, Swift RI, Tekkis PP , et al. Extramural venous invasion is a potential imaging predictive biomarker of neoadjuvant treatment in rectal cancer[J]. Br J Cancer, 2014,110(1):19-25.
doi: 10.1038/bjc.2013.603 |
| [9] |
Birlik B, Obuz F, Elibol FD , et al. Diffusion-weighted MRI and MR-volumetry: in the evaluation of tumor response after preoperative chemoradiotherapy in patients with locally advanced rectal cancer[J]. Magn Reson Imaging, 2015,33(2):201-212.
doi: 10.1016/j.mri.2014.08.041 |
| [10] |
McCawley N, Clancy C, O’Neill BD , et al. Mucinous rectal adenocarcinoma is associated with a poor response to neoadjuvant chemoradiotherapy: A systematic review and meta-analysis[J]. Dis Colon Rectum, 2016,59(12):1200-1208.
doi: 10.1097/DCR.0000000000000635 |
| [11] |
Agarwal A, Chang GJ, Hu CY , et al. Quantified pathologic response assessed as residual tumor burden is a predictor of recurrence-free survival in patients with rectal cancer who undergo resection after neoadjuvant chemoradiotherapy[J]. Cancer, 2013,119(24):4231-4241.
doi: 10.1002/cncr.28331 |
| [12] |
Maas M, Nelemans PJ, Valentini V , et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data[J]. Lancet Oncol, 2010,11(9):835-844.
doi: 10.1016/S1470-2045(10)70172-8 |
| [13] |
Gérard JP, Chamorey E, Gourgou-Bourgade S , et al. Clinical complete response (cCR) after neoadjuvant chemoradiotherapy and conservative treatment in rectal cancer. Findings from the ACCORD 12/PRODIGE 2 randomized trial[J]. Radiother Oncol, 2015,115(2):246-252.
doi: 10.1016/j.radonc.2015.04.003 |
| [14] |
Chen MB, Wu XY, Yu R , et al. P53 status as a predictive biomarker for patients receiving neoadjuvant radiation-based treatment: a meta-analysis in rectal cancer[J]. PLoS One, 2012,7(9):e45388.
doi: 10.1371/journal.pone.0045388 |
| [15] |
D’Angelo E, Zanon C, Sensi F , et al. miR-194 as predictive biomarker of responsiveness to neoadjuvant chemoradiotherapy in patients with locally advanced rectal adenocarcinoma[J]. J Clin Pathol, 2018,71(4):344-350.
doi: 10.1136/jclinpath-2017-204690 |
| [16] |
Agostini M, Zangrando A, Pastrello C , et al. A functional biological network centered on XRCC3: a new possible marker of chemoradiotherapy resistance in rectal cancer patients[J]. Cancer Biol Ther, 2015,16(8):1160-1171.
doi: 10.1080/15384047.2015.1046652 |
| [17] | Scott JG, Berglund A, Schell MJ , et al. A genome-based model for adjusting radiotherapy dose (GARD): a retrospective, cohort-based study[J]. Lancet Oncol, 2016,18(2):202-211. |
| [18] |
Regine WF, Winter KA, Abrams RA , et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial[J]. JAMA, 2008,299(9):1019-1026.
doi: 10.1001/jama.299.9.1019 |
| [19] |
Coveler AL, Richard P, Apisarnthanarax S , et al. Is there a best radiosensitizing agent in the treatment of locally advanced rectal cancer?[J]. Current Colorectal Cancer Reports, 2016,12(4):189-200.
doi: 10.1007/s11888-016-0324-7 |
| [20] |
Aschele C, Cionini L, Lonardi S , et al. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: pathologic results of the star-01 rando-mized phase Ⅲ trial[J]. J Clin Oncol, 2011,29(20):2773-2780.
doi: 10.1200/JCO.2010.34.4911 |
| [1] | 梁丽,李鑫,农琳,董颖,张继新,李东,李挺. 子宫内膜癌微卫星不稳定性分析: 微小微卫星变换的意义[J]. 北京大学学报(医学版), 2023, 55(2): 254-261. |
| [2] | 赖玉梅,李忠武,李欢,吴艳,时云飞,周立新,楼雨彤,崔传亮. 68例肛管直肠黏膜黑色素瘤临床病理特征及预后[J]. 北京大学学报(医学版), 2023, 55(2): 262-269. |
| [3] | 熊焰,张波,聂立功,吴世凯,赵虎,李东,邸吉廷. 胸部SMARCA4缺失性未分化肿瘤的病理诊断与联合免疫检测点抑制剂治疗[J]. 北京大学学报(医学版), 2023, 55(2): 351-356. |
| [4] | 丁婷婷,曾楚雄,胡丽娜,余明华. 基于癌症基因组图谱数据库结直肠癌免疫细胞浸润预测模型的建立[J]. 北京大学学报(医学版), 2022, 54(2): 203-208. |
| [5] | 张旭初,张建华,王荣福,范岩,付占立,闫平,赵光宇,白艳霞. 18F-FDG PET/CT联合多种肿瘤标志物在结直肠中分化腺癌术后复发及转移中的应用价值[J]. 北京大学学报(医学版), 2019, 51(6): 1071-1077. |
| [6] | 陈杨,王艳荣,石燕,戴广海. 晚期结直肠癌患者一线应用FOLFOX方案化疗引起中性粒细胞减少的预后价值[J]. 北京大学学报(医学版), 2017, 49(4): 669-674. |
| [7] | 刘艳霞,杨雪松,付卫,姚宏伟. 单核苷酸多态性位点rs6983267与溃疡性结肠炎及大肠癌的相关性[J]. 北京大学学报(医学版), 2016, 48(6): 994-999. |
| [8] | 司婧文, 王莉, 巴晓军, 张旭, 董颖, 张继新, 李文婷, 李挺. Lynch综合征临床病理筛查2例及文献回顾[J]. 北京大学学报(医学版), 2015, 47(5): 858-864. |
| [9] | 李志红, 刘丹, 何自静, 范志毅. 地塞米松对新辅助化疗后乳腺癌患者术后恶心呕吐发生率的影响[J]. 北京大学学报(医学版), 2015, 47(4): 685-689. |
| [10] | 原春辉,修典荣,葛辉玉,谭石,王行雁,张利,张同琳. 实时虚拟导航系统在结直肠癌肝转移射频消融治疗中的应用[J]. 北京大学学报(医学版), 2013, 45(6): 956-959. |
| [11] | 武颖超, 李沈, 汪欣, 刘玉村, 万远廉, 黄珊君. 淋巴结转移阴性直肠癌患者的预后因素分析[J]. 北京大学学报(医学版), 2012, 44(6): 937-941. |
| [12] | 李传凤, 李军, 白鹏, 吕愈敏. 结直肠腺瘤与患者血脂代谢水平[J]. 北京大学学报(医学版), 2011, 43(3): 432-435. |
| [13] | 汤坚强, 樊庆, 万远廉, 刘玉村, 汪欣, 吴涛, 潘义生, 吴问汉, 朱静. 组织因子/凝血因子Ⅶ在大肠癌中的异位表达及其临床意义[J]. 北京大学学报(医学版), 2009, 41(5): 531-536. |
| [14] | 张耀朋, 吕愈敏, 李军, 韩亚晶, 金珠, 李传凤. 过氧化物酶增殖物激活受体-γ在舒林酸干预治疗大鼠结直肠癌前病变中的作用[J]. 北京大学学报(医学版), 2009, 41(2): 168-173. |
| [15] | 汤坚强, 万远廉, 刘玉村, 戎龙, 汪欣, 吴涛, 潘义生, 朱静. 组织因子/活化凝血Ⅶ因子复合物对大肠癌LoVo细胞系表达基质金属蛋白酶-7的调控作用[J]. 北京大学学报(医学版), 2007, 39(5): 453-457. |
|
||