北京大学学报(医学版) ›› 2023, Vol. 55 ›› Issue (2): 351-356. doi: 10.19723/j.issn.1671-167X.2023.02.022
胸部SMARCA4缺失性未分化肿瘤的病理诊断与联合免疫检测点抑制剂治疗
熊焰1,*( ),张波2,聂立功3,吴世凯4,赵虎5,李东1,邸吉廷1
),张波2,聂立功3,吴世凯4,赵虎5,李东1,邸吉廷1
- 1. 北京大学第一医院病理科, 北京 100034
2. 北京大学基础医学院病理学系/北京大学第三医院病理科, 北京 100191
3. 北京大学第一医院呼吸与危重症学科, 北京 100034
4. 北京大学第一医院肿瘤科, 北京 100034
5. 北京大学第一医院胸外科, 北京 100034
Thoracic SMARCA4-deficient undifferentiated tumor-pathological diagnosis and combined immune checkpoint inhibitor treatment
Yan XIONG1,*( ),Bo ZHANG2,Li-gong NIE3,Shi-kai WU4,Hu ZHAO5,Dong LI1,Ji-ting DI1
),Bo ZHANG2,Li-gong NIE3,Shi-kai WU4,Hu ZHAO5,Dong LI1,Ji-ting DI1
- 1. Department of Pathology, Peking University First Hospital, Beijing 100034, China
2. Department of Pathology, School of Basic Medical Sciences Peking University/Peking University Third Hospital, Beijing 100191, China
3. Department of Respiratory and Critical Care Medicine, Peking University First Hospital, Beijing 100034, China
4. Department of Oncology, Peking University First Hospital, Beijing 100034, China
5. Department of Thoracic Surgery, Peking University First Hospital, Beijing 100034, China
摘要:
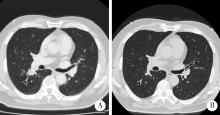
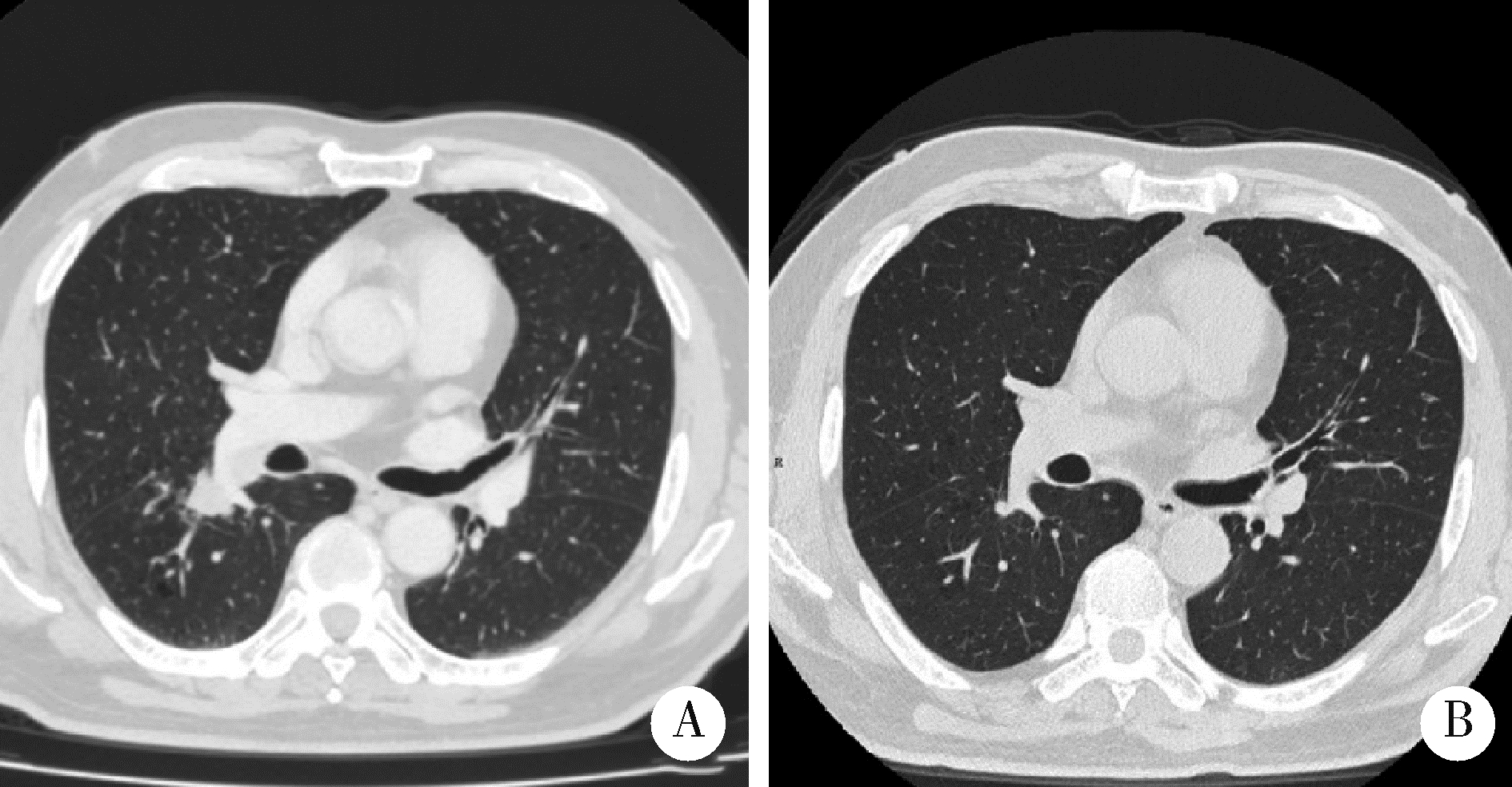
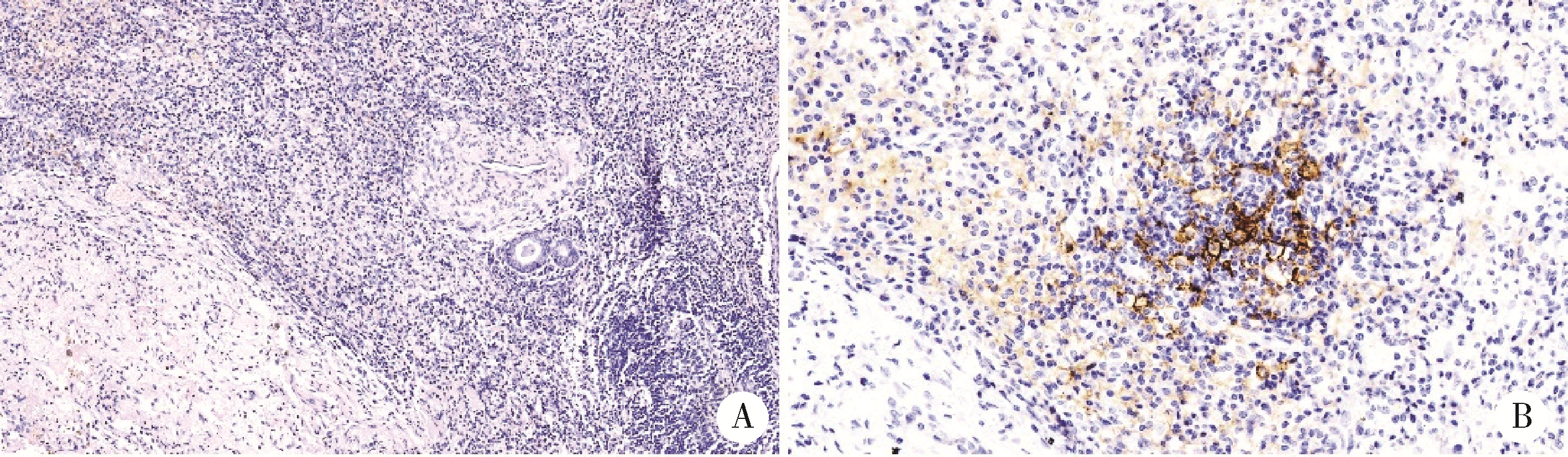
探讨胸部SMARCA4缺失性未分化肿瘤(SMARCA4-deficient undifferentiated tumor,SMARCA4-UT)的临床病理特点和治疗策略。SMARCA4-UT是2021版《世界卫生组织胸部肿瘤分类》中的新类型,各科医生对其诊断、治疗、预后等问题均较为陌生。本次多学科研讨会以北京大学第一医院收治的1例胸部SMARCA4-UT为例,集中研讨了该类肿瘤的病理诊断、分子检测、治疗相关分子靶点、免疫检验点抑制剂治疗以及新辅助治疗反应的病理学评估等问题。患者为老年男性,有长期吸烟史,以右肺下叶实性肿瘤快速进展入院。病理学检测,肿瘤细胞上皮样,组织结构上无任何分化特点;免疫组织化学染色显示,CD34弥漫阳性,SALL4部分细胞阳性,SMARCA4基因编码的蛋白BRG1表达缺失;二代测序证实,肿瘤存在SMARCA4基因突变(c.2196T>G,p.Y732Ter)。综上,病理诊断符合胸部SMARCA4-UT。术前TNM分期为T1N2M0(ⅢA)。程序性细胞死亡1-配体1(programmed cell death 1-ligand 1, PD-L1,克隆号SP263)抗体免疫组化染色显示,阳性肿瘤细胞比例评分(tumor proportion score,TPS)为2%。肿瘤突变负荷(tumor mutation burden, TMB)检测结果为16.3/Mb, 微卫星检测结果为微卫星稳定型(microsatellite stability, MSS)。经化疗联合免疫检测点抑制剂新辅助治疗两周后,行胸腔镜下右肺下叶切除术。术后新辅助治疗反应的病理学评估结果为病理学完全缓解(complete pathologic response,CPR),新辅助治疗后病理学分期为ypT0N0M0。术后完成了5周期化疗联合免疫检测点抑制剂辅助治疗。随访截止2022年10月,肿瘤无复发、转移,微小残留病灶(minimal residual disease,MRD)检测阴性。目前认为, 只要BRG1免疫组织化学染色阴性,无论是否检测到SMARCA4基因突变,均可归入SMARCA4缺失性肿瘤,SMARCA4缺失性肿瘤包括多种癌和肉瘤。SMARCA4-UT的诊断,除了BRG1表达缺失以外,还必须具备相应的组织形态,并排除其他伴有BRG1表达缺失的鳞癌、腺癌、大细胞癌等胸部常见恶性肿瘤。SMARCA4-UT恶性度高、总体预后差,几乎没有靶向治疗驱动基因突变,化疗效果差,而免疫检测点抑制剂是目前唯一显示疗效的药物。本病例是SMARCA4-UT病理诊断、分子检测和免疫检测点抑制剂治疗的一个成功案例,对于所有SMARCA4缺失性肿瘤的诊治都有启发意义。
中图分类号:
- R365
| 1 | Travis WD , Bubendorf L , Chung JH , et al. Thoracic SMARCA4-deficient undifferentiated tumor[M]. Lyon Cedex, France: International Agency for Research on Cancer (IARC), 2021: 111- 114. |
| 2 |
Nambirajan A , Jain D . Recent updates in thoracic SMARCA4-deficient undifferentiated tumor[J]. Semin Diagn Pathol, 2021, 38 (5): 83- 89.
doi: 10.1053/j.semdp.2021.06.001 |
| 3 |
Mittal P , Roberts CWM . The SWI/SNF complex in cancer: Biology, biomarkers and therapy[J]. Nat Rev Clin Oncol, 2020, 17 (7): 435- 448.
doi: 10.1038/s41571-020-0357-3 |
| 4 |
Mardinian K , Adashek JJ , Botta GP , et al. SMARCA4: Implications of an altered chromatin-remodeling gene for cancer development and therapy[J]. Mol Cancer Ther, 2021, 20 (12): 2341- 2351.
doi: 10.1158/1535-7163.MCT-21-0433 |
| 5 |
Alessi JV , Ricciuti B , Spurr LF , et al. SMARCA4 and other SWItch/Sucrose NonFermentable family genomic alterations in NSCLC: Clinicopathologic characteristics and outcomes to immune checkpoint inhibition[J]. J Thorac Oncol, 2021, 16 (7): 1176- 1187.
doi: 10.1016/j.jtho.2021.03.024 |
| 6 |
Sasaki M , Ogiwara H . Synthetic lethal therapy based on targeting the vulnerability of SWI/SNF chromatin remodeling complex-deficient cancers[J]. Cancer Sci, 2020, 111 (3): 774- 782.
doi: 10.1111/cas.14311 |
| 7 |
Travis WD , Dacic S , Wistuba I , et al. IASLC multidisciplinary recommendations for pathologic assessment of lung cancer resection specimens after neoadjuvant therapy[J]. J Thorac Oncol, 2020, 15 (5): 709- 740.
doi: 10.1016/j.jtho.2020.01.005 |
| 8 |
Provencio M , Nadal E , Insa A , et al. Neoadjuvant chemotherapy and nivolumab in resectable non small cell lung cancer (NADIM): An open-label, multicentre, single-arm, phase 2 trial[J]. Lancet Oncol, 2020, 21 (11): 1413- 1422.
doi: 10.1016/S1470-2045(20)30453-8 |
| 9 |
Liang W , Cai K , Chen C , et al. Expert consensus on neoadjuvant immunotherapy for non small cell lung cancer[J]. Transl Lung Cancer Res, 2020, 9 (6): 2696- 2715.
doi: 10.21037/tlcr-2020-63 |
| 10 |
Downes MR , Slodkowska E , Katabi N , et al. Inter-and intra-observer agreement of programmed death ligand 1 scoring in head and neck squamous cell carcinoma, urothelial carcinoma and breast carcinoma[J]. Histopathology, 2020, 76 (2): 191- 200.
doi: 10.1111/his.13946 |
| 11 |
Brueckl WM , Ficker JH , Zeitler G . Clinically relevant prognostic and predictive markers for immune-checkpoint-inhibitor (ICI) therapy in non-small cell lung cancer (NSCLC)[J]. BMC Can-cer, 2020, 20 (1): 1185.
doi: 10.1186/s12885-020-07690-8 |
| [1] | 李东,邸吉廷,熊焰. 程序性细胞死亡1-配体1在不同免疫组织化学染色方法的一致性比较[J]. 北京大学学报(医学版), 2023, 55(2): 339-342. |
| [2] | 刘菊梅,梁丽,张继新,戎龙,张梓怡,吴悠,赵旭东,李挺. 411例早期胃癌及癌前病变内镜黏膜下剥离术标本的病理学评估[J]. 北京大学学报(医学版), 2023, 55(2): 299-307. |
| [3] | 张威,叶颖江,任仙文,黄晶,申占龙. 利用转录组二代测序探索直肠癌术前放化疗的敏感性分子特征[J]. 北京大学学报(医学版), 2019, 51(3): 542-547. |
| [4] | 李志红, 刘丹, 何自静, 范志毅. 地塞米松对新辅助化疗后乳腺癌患者术后恶心呕吐发生率的影响[J]. 北京大学学报(医学版), 2015, 47(4): 685-689. |
|
||