北京大学学报(医学版) ›› 2022, Vol. 54 ›› Issue (2): 203-208. doi: 10.19723/j.issn.1671-167X.2022.02.001
• 论著 • 下一篇
基于癌症基因组图谱数据库结直肠癌免疫细胞浸润预测模型的建立
- 上海市浦东医院,复旦大学附属浦东医院肿瘤科,上海 201399
Establishment of a prediction model for colorectal cancer immune cell infiltration based on the cancer genome atlas (TCGA) database
DING Ting-ting,ZENG Chu-xiong,HU Li-na,YU Ming-hua( )
)
- Department of Oncology, Shanghai Pudong Hospital, Pudong Hospital Affiliated to Fudan University, Shanghai 201399, China
摘要:
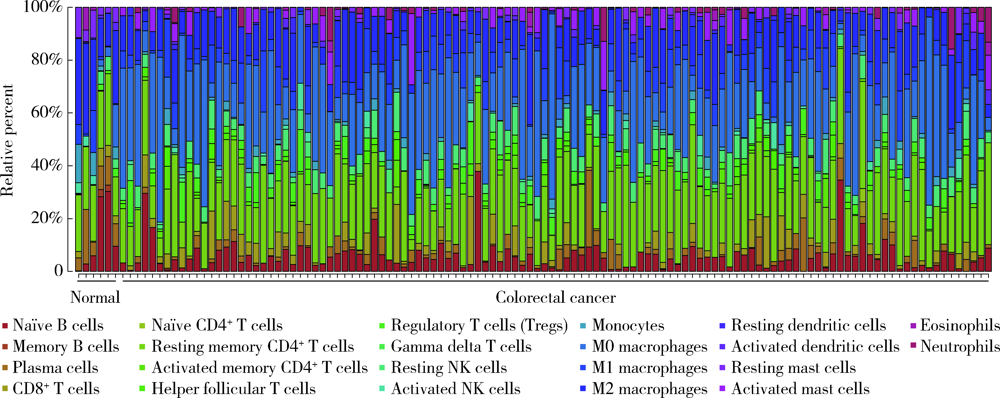
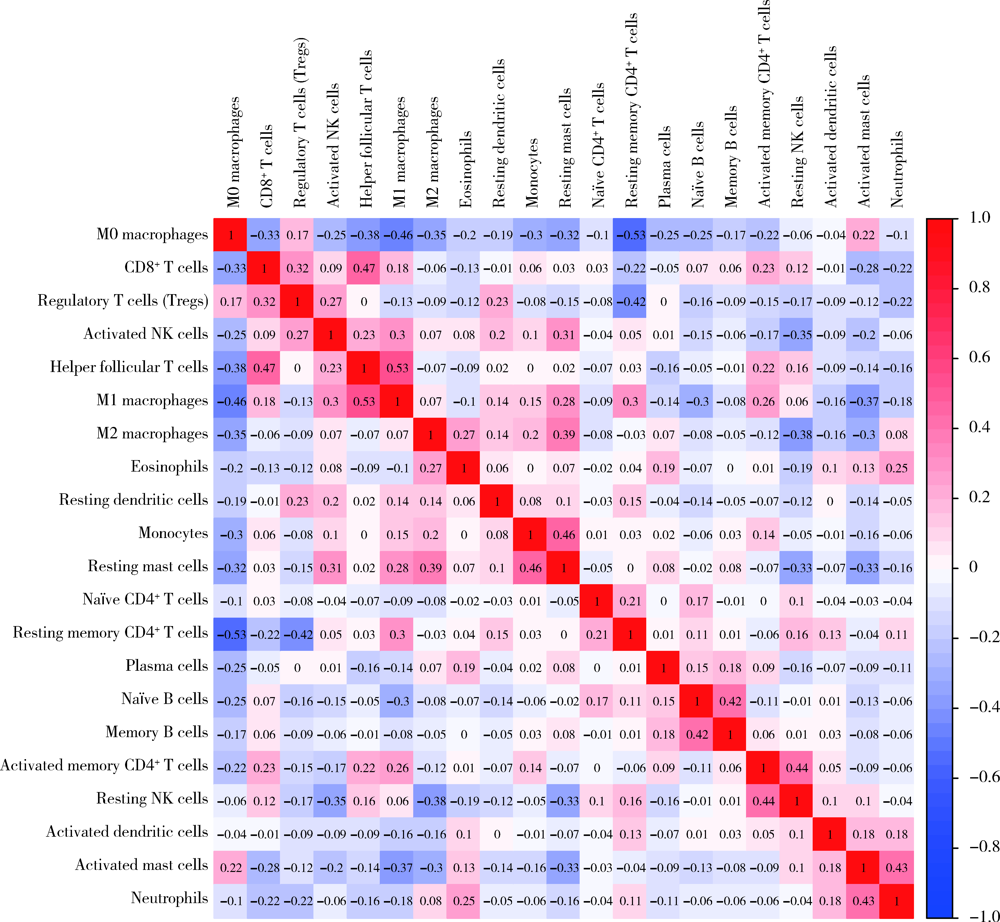
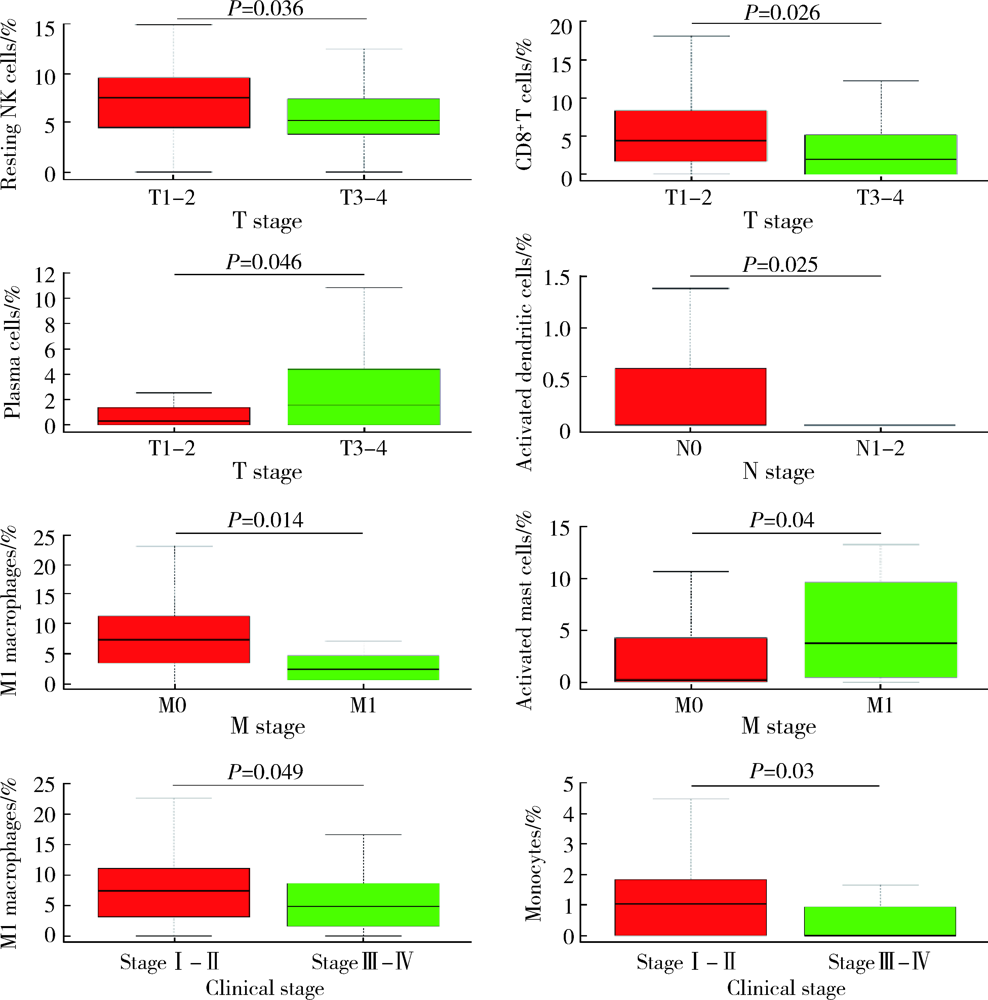
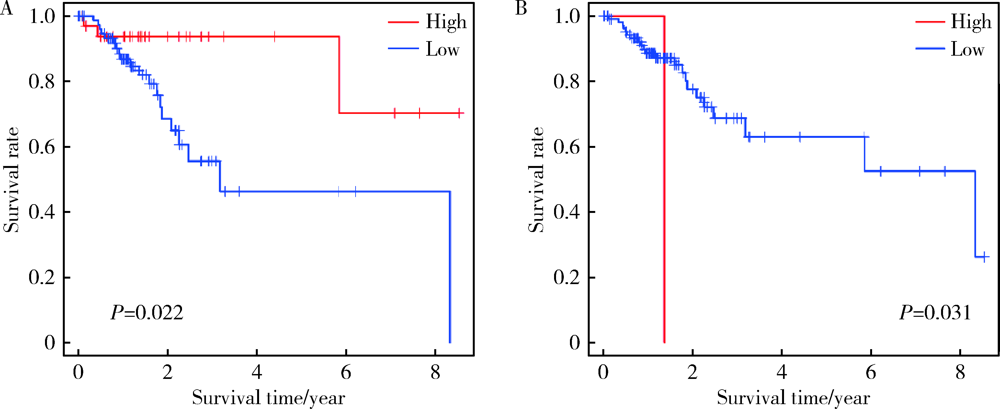
目的: 研究结直肠癌组织的免疫细胞浸润与临床预后之间的相关性。方法: 从癌症基因组图谱(the can-cer genome atlas,TCGA)中提取结直肠癌数据,基于反卷积算法(CIBERSORT)分析评估结直肠癌组织中22种肿瘤浸润性免疫细胞(tumor-infiltrating immune cells,TIICs)的浸润模式,以确定不同TIICs表达程度与5年生存率之间的关联。使用条形图展示结直肠癌样本中TIICs比例,绘制矩阵图分析不同TIICs之间的相关性。结果: 共从 TCGA数据库中提取了473例结直肠癌组织和41个正常对照组织,对比分析表明,结直肠癌组织中各种TIICs比例存在差异。在研究的细胞亚群中,结直肠癌组织中M0、M1和M2巨噬细胞和单核细胞的比例相对较高,而B细胞和中性粒细胞的比例相对较低。TIICs的比例与患者的TNM分期及临床分级显著相关:静息NK细胞、CD8+T细胞、浆细胞与T期相关,活化树突状细胞与N期相关,嗜酸性粒细胞、M1巨噬细胞及活化肥大细胞与M期相关,M1巨噬细胞和单核细胞与临床分级相关。生存分析结果显示,活化的树突状细胞与结直肠癌患者的5年生存率呈正相关,幼稚CD4+T细胞与5年生存率呈负相关。结论: 分析结直肠癌患者肿瘤组织TIICs亚群比例具有潜在的临床预后价值,可通过其识别可能从化疗中受益的患者,并预测新药的可能靶点。
中图分类号:
- R730.51
| [1] |
Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017[J]. CA Cancer J Clin, 2017, 67(3):177-193.
doi: 10.3322/caac.21395 |
| [2] | Shibutani M, Maeda K, Nagahara H, et al. Tumor-infiltrating lymphocytes predict the chemotherapeutic outcomes in patients with stage Ⅳ colorectal cancer[J]. In Vivo, 2018, 32(1):151-158. |
| [3] |
Guinney J, Dienstmann R, Wang X, et al. The consensus mole-cular subtypes of colorectal cancer[J]. Nat Med, 2015, 21(11):1350-1356.
doi: 10.1038/nm.3967 pmid: 26457759 |
| [4] |
Church J. Molecular genetics of colorectal cancer[J]. Sem Colon Rectal Surg, 2016, 27(4):172-175.
doi: 10.1053/j.scrs.2016.04.013 |
| [5] |
Bremnes RM, Al-Shibli K, Donnem T, et al. The role of tumor-infiltrating immune cells and chronic inflammation at the tumor site on cancer development, progression, and prognosis: Emphasis on non-small cell lung cancer[J]. J Thorac Oncol, 2011, 6(4):824-833.
doi: 10.1097/JTO.0b013e3182037b76 pmid: 21173711 |
| [6] |
Mao X, Xu J, Wang W, et al. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: New findings and future perspectives[J]. Mol Cancer, 2021, 20(1):131-142.
doi: 10.1186/s12943-021-01428-1 |
| [7] |
Baxevanis CN, Papamichail M, Perez SA. Immune classification of colorectal cancer patients: Impressive but how complete?[J]. Expert Opin Biol Ther, 2013, 13(4):517-526.
doi: 10.1517/14712598.2013.751971 |
| [8] |
Grizzi F, Basso G, Borroni EM, et al. Evolving notions on immune response in colorectal cancer and their implications for biomarker developmentc[J]. Inflamm Res, 2018, 67(5):375-389.
doi: 10.1007/s00011-017-1128-1 pmid: 29322204 |
| [9] |
Newman AM, Liu CL, Green MR, et al. Robust enumeration of cell subsets from tissue expression profiles[J]. Nat Methods, 2015, 12(5):453-457.
doi: 10.1038/nmeth.3337 pmid: 25822800 |
| [10] |
Liu X, Wu S, Yang Y, et al. The prognostic landscape of tumor-infiltrating immune cell and immunomodulators in lung cancer[J]. Biomed Pharmacother, 2017, 95:55-61.
doi: 10.1016/j.biopha.2017.08.003 |
| [11] |
Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis[J]. Nat Med, 2013, 19(11):1423-1437.
doi: 10.1038/nm.3394 |
| [12] |
Klemm F, Joyce JA. Microenvironmental regulation of therapeutic response in cancer[J]. Trends Cell Biol, 2015, 25(4):198-213.
doi: 10.1016/j.tcb.2014.11.006 |
| [13] |
Anitei MG, Zeitoun G, Mlecnik B, et al. Prognostic and predictive values of the immunoscore in patients with rectal cancer[J]. Clin Cancer Res, 2014, 20(7):1891-1899.
doi: 10.1158/1078-0432.CCR-13-2830 |
| [14] |
Huh JW, Lee JH, Kim HR. Prognostic significance of tumorinfiltrating lymphocytes for patients with colorectal cancer[J]. Arch Surg, 2012, 147(4):366-372.
doi: 10.1001/archsurg.2012.35 |
| [15] |
Karpinski P, Rossowska J, Sasiadek MM. Immunological landscape of consensus clusters in colorectal cancer[J]. Oncotarget, 2017, 8(62):105299-105311.
doi: 10.18632/oncotarget.v8i62 |
| [16] |
Mirjolet C, Charon-Barra C, Ladoire S, et al. Tumor lymphocyte immune response to preoperative radiotherapy in locally advanced rectal cancer: The LYMPHOREC study[J]. Oncoimmunology, 2018, 7(3):e1396402.
doi: 10.1080/2162402X.2017.1396402 |
| [17] |
Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome[J]. Science, 2006, 313(5759):1960-1964.
doi: 10.1126/science.1129139 |
| [18] |
Klintrup K, Makinen JM, Kauppila S, et al. Inflammation and prognosis in colorectal cancer[J]. Eur J Cancer, 2005, 41(17):2645-2654.
doi: 10.1016/j.ejca.2005.07.017 pmid: 16239109 |
| [19] |
Li T, Fan J, Wang B, et al. TIMER: A web server for comprehensive analysis of tumor-infiltrating immune cells[J]. Cancer Res, 2017, 77(21):e108-e110.
doi: 10.1158/0008-5472.CAN-17-0307 |
| [20] |
Pagès F, Mlecnik B, Marliot F, et al. International validation of the consensus immunoscore for the classification of colon cancer: A prognostic and accuracy study[J]. Lancet, 2018, 391(10135):2128-2139.
doi: 10.1016/S0140-6736(18)30789-X |
| [21] |
Steinman RM. Decisions about dendritic cells: Past, present, and future[J/OL]. Annu Rev Immunol, 2012, 30:1-22. doi: 10.1146/annurevimmunol-100311-102839.
doi: 10.1146/annurevimmunol-100311-102839 |
| [22] |
Shimizu K, Kotera Y, Aruga A, et al. Postoperative dendritic cell vaccine plus activated T-cell transfer improves the survival of patients with invasive hepatocellular carcinoma[J]. Hum Vaccin Immunother, 2014, 10(4):970-976.
doi: 10.4161/hv.27678 |
| [23] | Pagès F, Kirilovsky A, Mlecnik B, et al. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer[J]. J Clin Oncol, 2009, 27(35):5944-5951. |
| [24] |
Gannon PO, Baumgaertner P, Huber A, et al. Rapid and continued T-cell differentiation into long-term effector and memory stem cells in vaccinated melanoma patients[J]. Clin Cancer Res, 2017, 23(13):3285-3296.
doi: 10.1158/1078-0432.CCR-16-1708 |
| [1] | 刘园梅, 傅义程, 郝靖欣, 张福春, 刘慧琳. 老年髋部骨折患者住院期间发生术后心力衰竭的列线图预测模型的构建及验证[J]. 北京大学学报(医学版), 2024, 56(5): 874-883. |
| [2] | 李志存, 吴天俣, 梁磊, 范宇, 孟一森, 张骞. 穿刺活检单针阳性前列腺癌术后病理升级的危险因素分析及列线图模型构建[J]. 北京大学学报(医学版), 2024, 56(5): 896-901. |
| [3] | 欧俊永,倪坤明,马潞林,王国良,颜野,杨斌,李庚午,宋昊东,陆敏,叶剑飞,张树栋. 肌层浸润性膀胱癌合并中高危前列腺癌患者的预后因素[J]. 北京大学学报(医学版), 2024, 56(4): 582-588. |
| [4] | 刘帅,刘磊,刘茁,张帆,马潞林,田晓军,侯小飞,王国良,赵磊,张树栋. 伴静脉癌栓的肾上腺皮质癌的临床治疗及预后[J]. 北京大学学报(医学版), 2024, 56(4): 624-630. |
| [5] | 周泽臻,邓绍晖,颜野,张帆,郝一昌,葛力源,张洪宪,王国良,张树栋. 非转移性T3a肾细胞癌患者3年肿瘤特异性生存期预测[J]. 北京大学学报(医学版), 2024, 56(4): 673-679. |
| [6] | 柴晓东,孙子文,李海爽,朱靓怡,刘小旦,刘延涛,裴斐,常青. 髓母细胞瘤分子亚型中CD8+T淋巴细胞浸润的临床病理特点[J]. 北京大学学报(医学版), 2024, 56(3): 512-518. |
| [7] | 苏俊琪,王晓颖,孙志强. 舌鳞状细胞癌根治性切除术后患者预后预测列线图的构建与验证[J]. 北京大学学报(医学版), 2024, 56(1): 120-130. |
| [8] | 李建斌,吕梦娜,池强,彭一琳,刘鹏程,吴锐. 干燥综合征患者发生重症新型冠状病毒肺炎的早期预测[J]. 北京大学学报(医学版), 2023, 55(6): 1007-1012. |
| [9] | 薛子璇,唐世英,邱敏,刘承,田晓军,陆敏,董靖晗,马潞林,张树栋. 青年肾肿瘤伴瘤栓的临床病理特征及预后分析[J]. 北京大学学报(医学版), 2023, 55(5): 802-811. |
| [10] | 毛海,张帆,张展奕,颜野,郝一昌,黄毅,马潞林,褚红玲,张树栋. 基于MRI前列腺腺体相关参数构建腹腔镜前列腺癌术后尿失禁的预测模型[J]. 北京大学学报(医学版), 2023, 55(5): 818-824. |
| [11] | 卢汉,张建运,杨榕,徐乐,李庆祥,郭玉兴,郭传瑸. 下颌牙龈鳞状细胞癌患者预后的影响因素[J]. 北京大学学报(医学版), 2023, 55(4): 702-707. |
| [12] | 朱晓娟,张虹,张爽,李东,李鑫,徐玲,李挺. 人表皮生长因子受体2低表达乳腺癌的临床病理学特征及预后[J]. 北京大学学报(医学版), 2023, 55(2): 243-253. |
| [13] | 梁丽,李鑫,农琳,董颖,张继新,李东,李挺. 子宫内膜癌微卫星不稳定性分析: 微小微卫星变换的意义[J]. 北京大学学报(医学版), 2023, 55(2): 254-261. |
| [14] | 赖玉梅,李忠武,李欢,吴艳,时云飞,周立新,楼雨彤,崔传亮. 68例肛管直肠黏膜黑色素瘤临床病理特征及预后[J]. 北京大学学报(医学版), 2023, 55(2): 262-269. |
| [15] | 李敏,侯林卿,金月波,何菁. 系统性红斑狼疮合并视网膜病变的临床及免疫学特点[J]. 北京大学学报(医学版), 2022, 54(6): 1106-1111. |
|
||