北京大学学报(医学版) ›› 2021, Vol. 53 ›› Issue (4): 686-691. doi: 10.19723/j.issn.1671-167X.2021.04.011
多西他赛联合卡铂治疗转移性去势抵抗性前列腺癌的临床疗效
- 北京大学第一医院泌尿外科,北京大学泌尿外科研究所,国家泌尿、男性生殖系肿瘤研究中心, 北京 100034
Clinical efficacy of docetaxel combined with carboplatin in patients with metastatic castration-resistant prostate cancer
BAI Gao-chen,SONG Yi( ),JIN Jie(
),JIN Jie( ),YU Wei,HE Zhi-song
),YU Wei,HE Zhi-song
- National Urological Cancer Center, Beijing 100034, China
摘要:
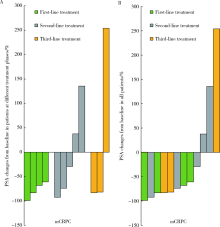
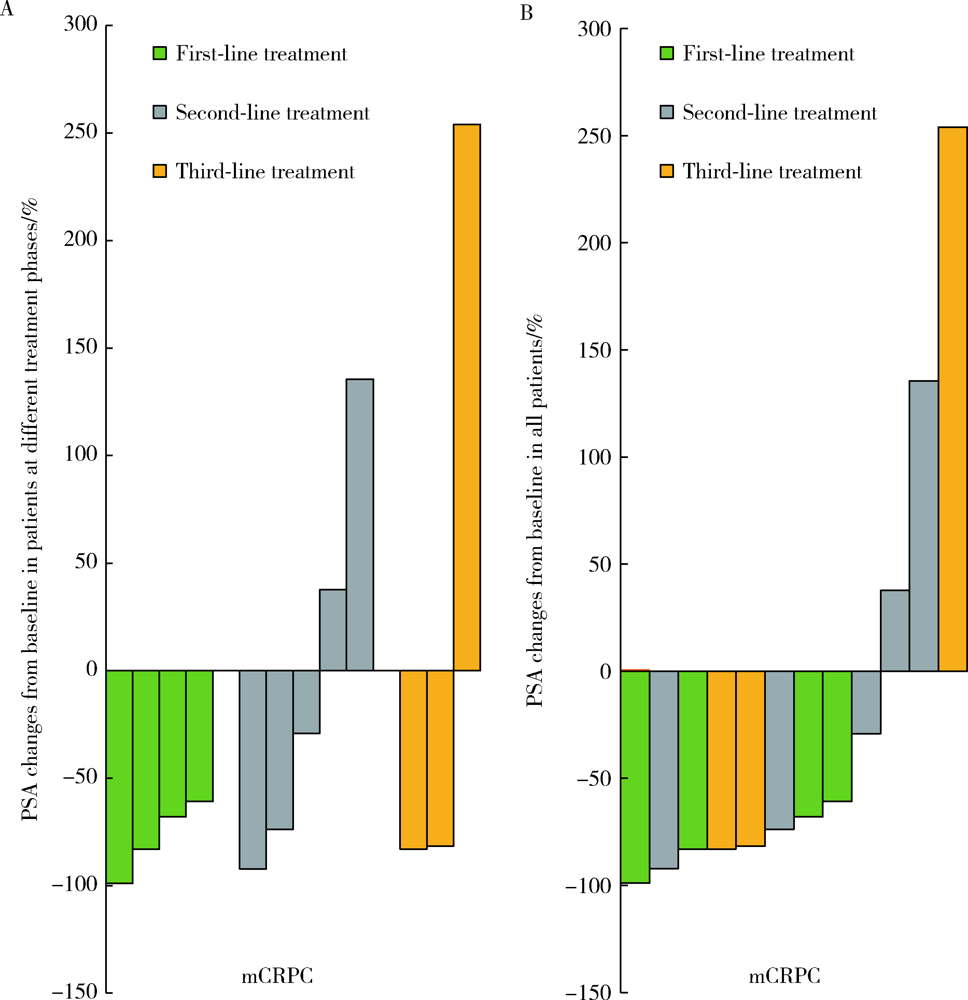
目的: 评估转移性去势抵抗性前列腺癌(metastatic castration-resistant prostate cancer, mCRPC)患者进行多西他赛联合卡铂治疗的近期疗效和安全性。方法: 选取 2017年5月至2019年7月在北京大学第一医院治疗的15例mCRPC患者为研究对象,中位年龄70岁(43~77岁),病理类型均为前列腺腺癌,影像学证实为全身转移,采用多西他赛联合卡铂的化疗方案,观察化疗4周期后前列腺特异性抗原(prostate-specific antigen, PSA)下降幅度、疼痛缓解率及不良反应发生情况,并进行数据分析。结果: 15例患者中,12例至少完成4个周期化疗并进行近期疗效评价,其中8例 PSA下降幅度>50%,有效率为66.7%;9例伴有骨痛的患者,疼痛数字评分法(numerical rating scales, NRS)平均分从4.7分下降至2.4分,有4例骨痛明显缓解,癌痛缓解率为44.4%;具有可测量转移病灶的4例患者中,达到部分缓解2例,疾病稳定1例,疾病进展1例;化疗的主要不良反应包括骨髓抑制、胃肠道反应、乏力、神经障碍等,大部分患者均在可耐受的范围。结论: 多西他赛联合卡铂治疗mCRPC具有良好的近期疗效,治疗过程中严重不良反应发生率较低,安全性较高,值得在临床上进一步推广和探索。
中图分类号:
- R697+.3
| [1] |
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020 [J]. CA Cancer J Clin, 2020, 70(1):7-30.
doi: 10.3322/caac.v70.1 |
| [2] |
Wu C, Li M, Meng H, et al. Analysis of status and counter-measures of cancer incidence and mortality in China [J]. Sci China Life Sci, 2019, 62(5):640-647.
doi: 10.1007/s11427-018-9461-5 |
| [3] |
Lowrance WT, Murad MH, Oh WK, et al. Castration-resistant prostate cancer: AUA guideline amendment 2018 [J]. J Urol, 2018, 200(6):1264-1272.
doi: S0022-5347(18)43671-3 pmid: 30086276 |
| [4] | 中国抗癌协会泌尿男生殖系肿瘤专业委员会. 2018版转移性前列腺癌诊治中国专家共识 [J]. 中华外科杂志, 2018, 56(9):646-652. |
| [5] |
Corn PG, Tu SM, Zurita AJ, et al. A multi-institutional ran-domized phase Ⅱ study (NCT01505868) of cabazitaxel (CAB) plus or minus carboplatin (CARB) in men with metastatic castration-resistant prostate cancer (mCRPC) [J]. J Clin Oncol, 2015, 33(Suppl 15):5010.
doi: 10.1200/jco.2015.33.15_suppl.5010 |
| [6] |
Aparicio AM, Harzstark AL, Corn PG, et al. Platinum-based chemotherapy for variant castrate-resistant prostate cancer [J]. Clin Cancer Res, 2013, 19(13):3621-3630.
doi: 10.1158/1078-0432.CCR-12-3791 |
| [7] |
Leal F, García-Perdomo HA. Effectiveness of platinum-based chemotherapy in patients with metastatic prostate cancer: systema-tic review and meta-analysis [J]. Clin Genitourin Cancer, 2019, 17(3):e627-e644.
doi: 10.1016/j.clgc.2019.03.008 |
| [8] |
Kantoff PW, Halabi S, Conaway M, et al. Hydrocortisone with or without mitoxantrone in men with hormone-refractory prostate can-cer: results of the cancer and leukemia group B 9182 study [J]. J Clin Oncol, 1999, 17(8):2506-2513.
pmid: 10561316 |
| [9] |
Tannock IF, Osoba D, Stockler MR, et al. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative end points [J]. J Clin Oncol, 1996, 14(6):1756-1764.
pmid: 8656243 |
| [10] |
Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer [J]. N Engl J Med, 2004, 351(15):1502-1512.
doi: 10.1056/NEJMoa040720 |
| [11] |
Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer [J]. N Engl J Med, 2004, 351(15):1513-1520.
doi: 10.1056/NEJMoa041318 |
| [12] |
Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer [J]. N Engl J Med, 2015, 373(8):737-746.
doi: 10.1056/NEJMoa1503747 |
| [13] |
James ND, Sydes MR, Clarke NW, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial [J]. Lancet, 2016, 387(10024):1163-1177.
doi: 10.1016/S0140-6736(15)01037-5 |
| [14] |
Oh WK, Tay MH, Huang J. Is there a role for platinum chemotherapy in the treatment of patients with hormone-refractory prostate cancer? [J]. Cancer, 2007, 109(3):477-486.
doi: 10.1002/(ISSN)1097-0142 |
| [15] |
Hager S, Ackermann CJ, Joerger M, et al. Anti-tumour activity of platinum compounds in advanced prostate cancer-a systematic literature review [J]. Ann Oncol, 2016, 27(6):975-984.
doi: S0923-7534(19)35660-1 pmid: 27052650 |
| [16] |
Huan SD, Stewart DJ, Aitken SE, et al. Combination of epirubicin and cisplatin in hormone-refractory metastatic prostate cancer [J]. Am J Clin Oncol, 1999, 22(5):471-474.
doi: 10.1097/00000421-199910000-00010 |
| [17] | Kaku H, Saika T, Tsushima T, et al. Combination chemotherapy with estramustine phosphate, ifosfamide and cisplatin for hormone-refractory prostate cancer [J]. Acta Med Okayama, 2006, 60(1):43-49. |
| [18] |
Dorff TB, Tsao-Wei DD, Groshen S, et al. Efficacy of oxaliplatin plus pemetrexed in chemotherapy pretreated metastatic castration-resistant prostate cancer [J]. Clin Genitourin Cancer, 2013, 11(4):416-422.
doi: 10.1016/j.clgc.2013.07.011 |
| [19] |
Gasent-Blesa JM, Giner-Marco V, Giner-Bosch V, et al. Phase Ⅱ trial of oxaliplatin and capecitabine after progression to first-line chemotherapy in androgen-independent prostate cancer patients [J]. Am J Clin Oncol, 2011, 34(2):155-159.
doi: 10.1097/COC.0b013e3181d6b453 |
| [20] |
Flaig TW, Barqawi A, Miller G, et al. A phase Ⅱ trial of dexa-methasone, vitamin D, and carboplatin in patients with hormone-refractory prostate cancer [J]. Cancer, 2006, 107(2):266-274.
doi: 10.1002/(ISSN)1097-0142 |
| [21] | Oh WK, Halabi S, Kelly WK, et al. A phase Ⅱ study of estramustine, docetaxel, and carboplatin with granulocyte-colony-stimulating factor support in patients with hormone-refractory prostate carcinoma: Cancer and Leukemia Group B 99813 [J]. Can-cer, 2003, 98(12):2592-2598. |
| [22] |
Vlachostergios PJ, Papandreou CN. Targeting neuroendocrine prostate cancer: molecular and clinical perspectives [J]. Front Oncol, 2015, 5:6.
doi: 10.3389/fonc.2015.00006 pmid: 25699233 |
| [23] |
Pomerantz MM, Spisák S, Jia L, et al. The association between germline BRCA2 variants and sensitivity to platinum-based chemotherapy among men with metastatic prostate cancer [J]. Cancer, 2017, 123(18):3532-3539.
doi: 10.1002/cncr.30808 pmid: 28608931 |
| [24] |
Mateo J, Carreira S, Sandhu S, et al. DNA-repair defects and olaparib in metastatic prostate cancer [J]. N Engl J Med, 2015, 373(18):1697-1708.
doi: 10.1056/NEJMoa1506859 |
| [25] |
Ross RW, Beer TM, Jacobus S, et al. A phase 2 study of carbo-platin plus docetaxel in men with metastatic hormone-refractory prostate cancer who are refractory to docetaxel [J]. Cancer, 2008, 112(3):521-526.
doi: 10.1002/(ISSN)1097-0142 |
| [26] |
Kentepozidis N, Soultati A, Giassas S, et al. Paclitaxel in combination with carboplatin as salvage treatment in patients with castration-resistant prostate cancer: a Hellenic oncology research group multicenter phase Ⅱ study [J]. Cancer Chemother Pharmacol, 2012, 70(1):161-168.
doi: 10.1007/s00280-012-1896-9 |
| [27] |
Carlin BI, Andriole GL. The natural history, skeletal complications, and management of bone metastases in patients with prostate carcinoma [J]. Cancer, 2000, 88(Suppl 12):2989-2994.
doi: 10.1002/(ISSN)1097-0142 |
| [28] | Jocham D, Sommerauer M. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomized open-label trial [J]. Eur Urol, 2011, 59(4):659. |
| [1] | 于书慧,韩佳凝,钟丽君,陈聪语,肖云翔,黄燕波,杨洋,车新艳. 术前盆底肌电生理参数对前列腺癌根治性切除术后早期尿失禁的预测价值[J]. 北京大学学报(医学版), 2024, 56(4): 594-599. |
| [2] | 颜野,李小龙,夏海缀,朱学华,张羽婷,张帆,刘可,刘承,马潞林. 前列腺癌根治术后远期膀胱过度活动症的危险因素[J]. 北京大学学报(医学版), 2024, 56(4): 589-593. |
| [3] | 邢念增,王明帅,杨飞亚,尹路,韩苏军. 前列腺免活检创新理念的临床实践及其应用前景[J]. 北京大学学报(医学版), 2024, 56(4): 565-566. |
| [4] | 黄教悌,胡菁,韩博. 治疗相关神经内分泌前列腺癌机制研究与靶向治疗新进展[J]. 北京大学学报(医学版), 2024, 56(4): 557-561. |
| [5] | 刘圣杰,侯惠民,吕政通,丁鑫,王璐,张磊,刘明. 双极雄激素序贯免疫检查点抑制剂治疗转移性去势抵抗性前列腺癌4例[J]. 北京大学学报(医学版), 2022, 54(4): 766-769. |
| [6] | 徐涛,韩敬丽,姚伟娟. 雄激素剥夺治疗相关心血管疾病的机制与临床对策[J]. 北京大学学报(医学版), 2020, 52(4): 607-609. |
| [7] | 孙奎霞,闫存玲,李志艳,刘平,张伟,何群. 前列腺特异性抗原同源异构体2及其衍生指标在预测前列腺癌病理分级中的价值[J]. 北京大学学报(医学版), 2020, 52(2): 234-239. |
| [8] | 李文卿,任思楣,龙星博,田雨青. 棕榈酰化蛋白质组学分析揭示前列腺癌细胞中雄激素促进代谢相关蛋白棕榈酰化修饰[J]. 北京大学学报(医学版), 2020, 52(2): 227-233. |
| [9] | 张宽根,周雨禾,邵雅昆,梅放,由江峰,刘北英,裴斐. 肿瘤转移抑制基因LASS2/TMSG1 S248A突变体通过增加ATP6V0C表达促进前列腺癌的侵袭[J]. 北京大学学报(医学版), 2019, 51(2): 210-220. |
| [10] | 唐旭,赵卫红,宋琴琴,殷华奇,杜依青,盛正祚,王强,张晓威,李清,刘士军,徐涛. SOX10对前列腺癌细胞增殖及侵袭的影响[J]. 北京大学学报(医学版), 2018, 50(4): 602-606. |
| [11] | 邹鹏程,杨一峰,徐晓艳,刘北英,梅放,由江峰,刘启忱,裴斐 . 沉默液泡型ATP酶c亚基ATP6V0C抑制人前列腺癌细胞侵袭的分子机制[J]. 北京大学学报(医学版), 2017, 49(6): 937-947. |
| [12] | 纪光杰,黄聪,宋刚,李学松,宋毅,周利群. 去势抵抗性前列腺癌进展时间的预测因素分析[J]. 北京大学学报(医学版), 2017, 49(4): 657-662. |
| [13] | 杨恺惟, 虞巍, 宋毅, 黄立华, 韩文科, 何志嵩, 金杰, 周利群. 影响多西他赛联合泼尼松治疗转移性去势抵抗性前列腺癌疗效的因素分析[J]. 北京大学学报(医学版), 2015, 47(4): 592-596. |
| [14] | 余靖, 邸立军, 宋国红, 车利, 姜晗昉, 祝毓琳, 梁旭, 贾军, 张洁, 杨化兵, 王小利, 周心娜, 任军. 多西他赛联合塞替派与多西他赛联合卡培他滨治疗转移性乳腺癌的随机、对照临床研究[J]. 北京大学学报(医学版), 2011, 43(1): 151-156. |
| [15] | 刘汀, 王霄英, 王义. 用Cox模型对前列腺癌相关临床检查的多因素生存分析[J]. 北京大学学报(医学版), 2009, 41(2): 184-187. |
|
||