北京大学学报(医学版) ›› 2019, Vol. 51 ›› Issue (2): 210-220. doi: 10.19723/j.issn.1671-167X.2019.02.003
肿瘤转移抑制基因LASS2/TMSG1 S248A突变体通过增加ATP6V0C表达促进前列腺癌的侵袭
张宽根1,周雨禾1,邵雅昆1,梅放1,由江峰1,刘北英2,裴斐1,3,∆( )
)
- 1. 北京大学基础医学院病理学系, 北京 100191
2. 北京科技大学机械学院, 北京 100083
3. 北京大学第三医院病理科, 北京 100191
Novel tumor metastasis suppressorgene LASS2/TMSG1 S248A mutant promotes invasion of prostate cancer cells through increasing ATP6V0C expression
Kuan-gen ZHANG1,Yu-he ZHOU1,Ya-kun SHAO1,Fang MEI1,Jiang-feng YOU1,Bei-ying LIU2,Fei PEI1,3,∆( )
)
- 1. Department of Pathology, Peking University School of Basic Medical Sciences, Beijing 100191China
2. School of Mechanical Engineering, University of Science & Technology Beijing, Beijing 100083 China;
3. Department of Pathology, Peking University Third Hospital, Beijing 100191,China
摘要:
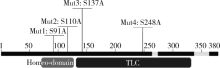
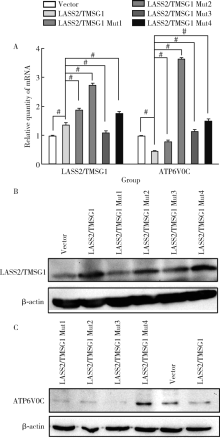
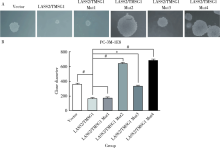
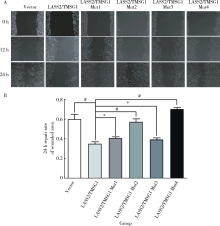
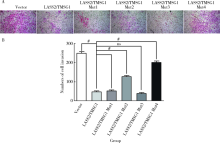
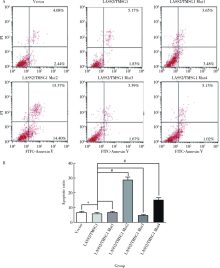
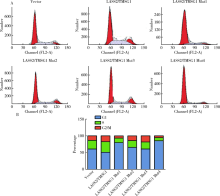
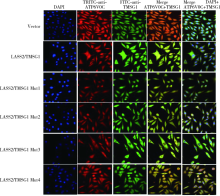
目的: 探讨LASS2/TMSG1及其突变体在前列腺癌细胞增殖、迁移和侵袭中的分子作用机制。方法: 构建表达LASS2/TMSG1全长及其4个突变体的pcDNA3真核表达载体,并稳定转染到高转移潜能前列腺癌PC-3M-1E8细胞系中;采用qPCR和Western blot法鉴定稳定转染效果,并分析不同点突变体对LASS2/TMSG1和ATP6V0C表达量的影响;采用生长曲线测定、四甲基偶氮唑蓝(MTT)掺入实验、软琼脂集落形成实验、细胞划痕修复实验、Matrigel穿膜侵袭实验和流式细胞术研究LASS2/TMSG1及其4个点突变体的细胞生物学功能,并通过免疫双重荧光染色分析LASS2/TMSG1不同突变体和ATP6V0C的相互作用情况。结果: qPCR和Western blot检测显示LASS2/TMSG1 S248A组较LASS2/TMSG1野生型组ATP6V0C表达增加了3倍(P<0.05), 且免疫双重荧光染色结果显示LASS2/TMSG1 S248A组ATP6V0C表达明显增加;与LASS2/TMSG1野生型组相比,LASS2/TMSG1 S248A组的细胞增殖能力、锚着不依赖生长能力、细胞迁移能力 (细胞迁移率从35.3%±3.2%增加到70.3%±3%)和侵袭能力(穿膜细胞数从50.0±3.2增加到203.0±6.5)明显提高(P<0.05), G0/G1期比例增加(从51.0%增加到85.4%,P<0.05),但细胞凋亡率亦明显升高(从7%增加到15.1%,P<0.05)。结论: LASS2 /TMSG1第248位丝氨酸突变为丙氨酸后(S248A)能促进前列腺癌细胞增殖、迁移和侵袭能力,其分子机制可能是LASS2/TMSG S248A使ATP6V0C表达量增加,进而促进前列腺癌的侵袭,提示LASS2/TMSG1蛋白第248位丝氨酸是抑制前列腺癌侵袭的重要功能位点。
中图分类号:
- R737.25
| [1] |
Cronin KA, Lake AJ, Scott S , et al. Annual Report to the nation on the status of cancer, part Ⅰ: national cancer statistics[J]. Cancer, 2018,124(13):2785-2800.
doi: 10.1002/cncr.31551 |
| [2] | 刘宇欣, 郑杰, 方伟岗 , 等. 具有不同转移潜能的前列腺癌细胞亚系的分离鉴定[J]. 中华病理学杂志, 1999,28(5):361-364. |
| [3] | 马春树, 刘宇欣, 郑杰 , 等. 应用mRNA差异显示技术克隆肿瘤转移相关基因LASS2/TMSG1[J]. 中国科学, 2002,32(3):270-275. |
| [4] |
Strausberg RL, Feingold EA, Grouse LH , et al. Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences[J]. Proc Natl Acad Sci USA, 2002,99(26):16899-16903.
doi: 10.1073/pnas.242603899 |
| [5] |
Pan H, Qin WX, Huo KK , et al. Cloning, mapping, and charaterization of a human homologue of the yeast longevity assurance gene LAG1[J]. Genomics, 2001,77(1/2):58-64.
doi: 10.1006/geno.2001.6614 |
| [6] |
Pewzner-Jung Y, Ben Dor S, Futerman AH . When do Lasses (longevity assurance genes) become CerS (ceramide synthases)? Insights into the regulation of ceramide synjournal[J]. J Biol Chem, 2006,281(35):25001-25005.
doi: 10.1074/jbc.R600010200 |
| [7] | 裴裴, 由江峰, 宁钧宇 , 等. 人肿瘤转移抑制基因TMSG-1单克隆抗体的制备、鉴定及在肿瘤检测中的应用[J]. 中华病理学杂志, 2005,34(1):15-21. |
| [8] |
Yu W, Wang L, Pei F , et al. A novel tumor metastasis suppressor gene LASS2/TMSG1 interacts with vacuolar ATPase through its homeodomain[J]. J Cell Biochem, 2013,114(3):570-583.
doi: 10.1002/jcb.v114.3 |
| [9] |
Ohta T, Numata M, Yagishita H , et al. Expression of 16 kDa proteolipid of vacuolar-type H(+)-ATPase in human pancreatic cancer[J]. Br J Cancer, 1996,73(12):1511-1517.
doi: 10.1038/bjc.1996.285 |
| [10] |
Sennoune SR, Bakunts K, Martinez GM , et al. Vacuolar H+-ATPase in human breast cancer cells with distinct metastatic potential: distribution and functional activity[J]. Am J Physiol Cell Physiol, 2004,286(6):1443-1452.
doi: 10.1152/ajpcell.00407.2003 |
| [11] |
Lee I, Skinner MA, Guo HB , et al. Expression of the vacuolar H+-ATPase 16-kDa subunit results in the Triton X-100-insoluble aggregation of beta1 integrin and reduction of its cell surface expression[J]. J Biol Chem, 2004,279(51):53007-53014.
doi: 10.1074/jbc.M405717200 |
| [12] |
Vitavska O, Wieczorek H, Merzendorfer H . A novel role for subunit C in mediating binding of the H+-V-ATPase to the actin cytoskeleton[J]. J Biol Chem, 2003,278(20):18499-18505.
doi: 10.1074/jbc.M212844200 |
| [13] |
Holliday LS, Lu M, Lee BS , et al. The amino-terminal domain of the B subunit of vacuolar H+-ATPase contains a filamentous actin binding site[J]. J Biol Chem, 2000,275(41):32331-32337.
doi: 10.1074/jbc.M004795200 |
| [14] |
Goldstein DJ, Andresson T, Sparkowski JJ , et al. The BPV-1 E5 protein, the 16 kDa membrane pore-forming protein and the PDGF receptor exist in a complex that is dependent on hydrophobic transmembrane interactions[J]. EMBO J, 1992,11(13):4851-4859.
doi: 10.1002/embj.1992.11.issue-13 |
| [15] |
Skinner MA , Wildeman AG. beta(1) integrin binds the 16-kDa subunit of vacuolar H(+)-ATPase at a site important for human papillomavirus E5 and platelet-derived growth factor signaling[J]. J Biol Chem, 1999,274(33):23119-23127.
doi: 10.1074/jbc.274.33.23119 |
| [16] |
Liotta LA, Kohn EC . The microenvironment of the tumour-host interface[J]. Nature, 2001,411(6835):375-379.
doi: 10.1038/35077241 |
| [17] | Zou P, Yang Y, Pei F , et al. Silencing of vacuolar ATPase c subunit ATP6V0C inhibits invasion of prostate cancer cell through LASS2/TMSG1 independent manner[J]. Oncol Rep, 2018,39(1):298-306. |
| [18] |
Kim SS, Chae HS, Bach JH , et al. P53 mediates ceramide-induced apoptosis in SKN-SH cells[J]. Oncogene, 2002,21(13):2020-2028.
doi: 10.1038/sj.onc.1205037 |
| [19] |
Kim HJ, Ghil KC, Kim MS , et al. Potentiation of ceramide-induced apoptosis by p27kip1 overexpression[J]. Arch Pharm Res, 2005,28(1):87-92.
doi: 10.1007/BF02975141 |
| [20] |
Yang H, Sadda MR, Li M , et al. S-adenosylmethionine and its metabolite induce apoptosis in HepG2 cells: Role of protein phosphatase 1 and Bcl-x(S)[J]. Hepatology, 2004,40(1):221-231.
doi: 10.1002/hep.v40:1 |
| [21] |
Lee JY, Bielawska AE, Obeid LM . Regulation of cyclin-depen-dent kinase 2 activity by ceramide[J]. Exp Cell Res, 2000,261(2):303-311.
doi: 10.1006/excr.2000.5028 |
| [22] |
Zhu XF, Liu ZC, Xie BF , et al. Ceramide induces cell cycle arrest and upregulates p27kip in nasopharyngeal carcinoma cells[J]. Cancer Lett, 2003,193(2):149-154.
doi: 10.1016/S0304-3835(03)00050-8 |
| [1] | 黄教悌,胡菁,韩博. 治疗相关神经内分泌前列腺癌机制研究与靶向治疗新进展[J]. 北京大学学报(医学版), 2024, 56(4): 557-561. |
| [2] | 邢念增,王明帅,杨飞亚,尹路,韩苏军. 前列腺免活检创新理念的临床实践及其应用前景[J]. 北京大学学报(医学版), 2024, 56(4): 565-566. |
| [3] | 颜野,李小龙,夏海缀,朱学华,张羽婷,张帆,刘可,刘承,马潞林. 前列腺癌根治术后远期膀胱过度活动症的危险因素[J]. 北京大学学报(医学版), 2024, 56(4): 589-593. |
| [4] | 于书慧,韩佳凝,钟丽君,陈聪语,肖云翔,黄燕波,杨洋,车新艳. 术前盆底肌电生理参数对前列腺癌根治性切除术后早期尿失禁的预测价值[J]. 北京大学学报(医学版), 2024, 56(4): 594-599. |
| [5] | 刘圣杰,侯惠民,吕政通,丁鑫,王璐,张磊,刘明. 双极雄激素序贯免疫检查点抑制剂治疗转移性去势抵抗性前列腺癌4例[J]. 北京大学学报(医学版), 2022, 54(4): 766-769. |
| [6] | 白杲琛,宋毅,金杰,虞巍,何志嵩. 多西他赛联合卡铂治疗转移性去势抵抗性前列腺癌的临床疗效[J]. 北京大学学报(医学版), 2021, 53(4): 686-691. |
| [7] | 徐涛,韩敬丽,姚伟娟. 雄激素剥夺治疗相关心血管疾病的机制与临床对策[J]. 北京大学学报(医学版), 2020, 52(4): 607-609. |
| [8] | 孙奎霞,闫存玲,李志艳,刘平,张伟,何群. 前列腺特异性抗原同源异构体2及其衍生指标在预测前列腺癌病理分级中的价值[J]. 北京大学学报(医学版), 2020, 52(2): 234-239. |
| [9] | 李文卿,任思楣,龙星博,田雨青. 棕榈酰化蛋白质组学分析揭示前列腺癌细胞中雄激素促进代谢相关蛋白棕榈酰化修饰[J]. 北京大学学报(医学版), 2020, 52(2): 227-233. |
| [10] | 唐旭,赵卫红,宋琴琴,殷华奇,杜依青,盛正祚,王强,张晓威,李清,刘士军,徐涛. SOX10对前列腺癌细胞增殖及侵袭的影响[J]. 北京大学学报(医学版), 2018, 50(4): 602-606. |
| [11] | 邹鹏程,杨一峰,徐晓艳,刘北英,梅放,由江峰,刘启忱,裴斐 . 沉默液泡型ATP酶c亚基ATP6V0C抑制人前列腺癌细胞侵袭的分子机制[J]. 北京大学学报(医学版), 2017, 49(6): 937-947. |
| [12] | 纪光杰,黄聪,宋刚,李学松,宋毅,周利群. 去势抵抗性前列腺癌进展时间的预测因素分析[J]. 北京大学学报(医学版), 2017, 49(4): 657-662. |
| [13] | 杨恺惟, 虞巍, 宋毅, 黄立华, 韩文科, 何志嵩, 金杰, 周利群. 影响多西他赛联合泼尼松治疗转移性去势抵抗性前列腺癌疗效的因素分析[J]. 北京大学学报(医学版), 2015, 47(4): 592-596. |
| [14] | 李伟军, 邢晓芳, 曲立科, 孟麟, 寿成超. PRL-3基因C104S位点突变体和CAAX缺失体的构建及表达[J]. 北京大学学报(医学版), 2009, 41(5): 516-520. |
| [15] | 刘汀, 王霄英, 王义. 用Cox模型对前列腺癌相关临床检查的多因素生存分析[J]. 北京大学学报(医学版), 2009, 41(2): 184-187. |
|
||