北京大学学报(医学版) ›› 2019, Vol. 51 ›› Issue (2): 221-227. doi: 10.19723/j.issn.1671-167X.2019.02.004
miR-106b-5p在调节内皮细胞基因表达谱中的作用
- 北京大学人民医院心血管内科,急性心肌梗死早期预警和干预北京市重点实验室,北京大学人民医院心血管转化医学研究中心, 北京 100044
Role of miR-106b-5p in the regulation of gene profiles in endothelial cells
Jing ZHANG,Su-fang LI,Hong CHEN( ),Jun-xian SONG
),Jun-xian SONG
- Center for Cardiovascular Translational Research, Peking University People’s Hospital, Beijing 100044, China;
摘要:
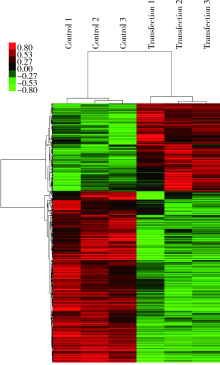
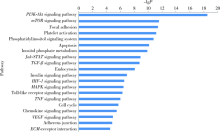
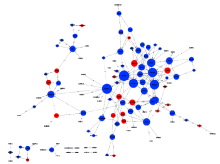
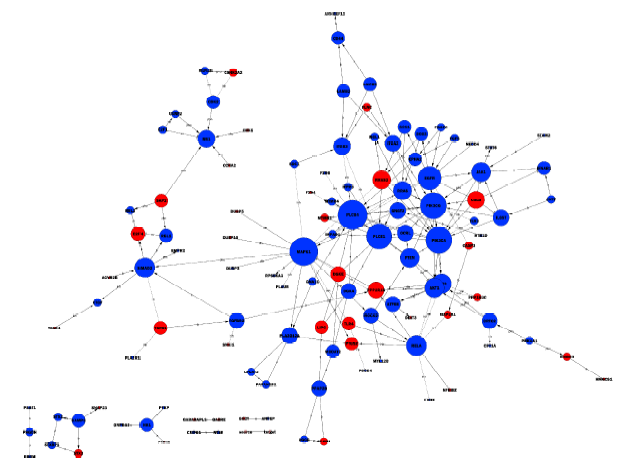
目的: 评估miR-106b-5p对内皮细胞基因表达谱的影响。方法: 对动脉粥样硬化组(n=9)及对照组(n=9)血浆中miRNAs表达谱进行筛查,采用共表达网络分别对两组样本全体miRNAs 的共表达模式进行分析。选取从两组网络中得到的共表达地位差异最为显著的miR-106b-5p进一步研究,通过转染miR-106b-5p mimics上调人脐静脉内皮细胞的miR-106b-5p表达水平,筛查转染后差异基因表达谱,并进一步通过日本京都基因与基因组百科全书(Kyoto Encyclopedia of Genes and Genomes,KEGG)信号转导通路数据库对差异基因富集的信号通路进行分析。结果: 动脉粥样硬化组患者血浆中miRNAs的共表达模式(140个节点,1 154条连接线)与对照组(140个节点,612条连接线)相比存在明显差异,在过表达miR-106b-5p后人脐静脉内皮细胞有746个基因水平发生了显著变化(组间差异倍数≥1.5,芯片错误发现率<0.01),主要包括磷脂酰肌醇-3激酶(phosphoinositide 3-kinase,PI3K)/蛋白激酶B(protein kinase B,PKB,又称Akt)信号通路、哺乳动物雷帕霉素受体蛋白(mammalian target of rapamycin,mTOR)信号通路、转化生长因子-β(transforming growth factor-β,TGF-β)信号通路、酪氨酸激酶-信号转导及转录激活因子信号通路(janus kinase / signal transducer and activator of transcription,Jak-STAT)信号通路、肿瘤坏死因子(tumor necrosis factor,TNF)信号通路、toll样受体(toll-like receptor,TLR)信号通路、血管内皮生长因子(vascular endothelial growth factor,VEGF)信号通路等20个信号通路。结论: 动脉粥样硬化患者血浆中miRNAs共表达模式发生了显著变化,其中共表达地位差异最为显著的miR-106b-5p可靶向调节血管内皮细胞多个信号通路。
中图分类号:
- R541.4
| [1] |
Bartel DP . MicroRNAs: genomics, biogenesis, mechanism, and function[J]. Cell, 2004,116(2):281-297.
doi: 10.1016/S0092-8674(04)00045-5 |
| [2] |
Small EM, Olson EN . Pervasive roles of microRNAs in cardiovascular biology[J]. Nature, 2011,469(7330):336-342.
doi: 10.1038/nature09783 |
| [3] |
Ren J, Zhang J, Xu N , et al. Signature of circulating microRNAs as potential biomarkers in vulnerable coronary artery disease[J]. PLoS One, 2013,8(12):e80738.
doi: 10.1371/journal.pone.0080738 |
| [4] |
Li P, Shen M, Gao F , et al. An antagomir to microRNA-106b-5p ameliorates cerebral ischemia and reperfusion injury in rats via inhibiting apoptosis and oxidative stress[J]. Mol Neurobiol, 2017,54(4):2901-2921.
doi: 10.1007/s12035-016-9842-1 |
| [5] |
Li N, Miao Y, Shan Y , et al. MiR-106b and miR-93 regulate cell progression by suppression of PTEN via PI3K/Akt pathway in breast cancer[J]. Cell Death Dis, 2017,8(5):e2796.
doi: 10.1038/cddis.2017.119 |
| [6] |
Wang J, Haubrock M, Cao KM , et al. Regulatory coordination of clustered microRNAs based on microRNA-transcription factor regulatory network[J]. BMC Syst Biol, 2011,5(12):199.
doi: 10.1186/1752-0509-5-199 |
| [7] |
Prieto C, Risueño A, Fontanillo C , et al. Human gene coexpression landscape: confident network derived from tissue transcriptomic profiles[J]. PLoS One, 2008,3(12):e3911.
doi: 10.1371/journal.pone.0003911 |
| [8] |
Zhang J, Li SF, Chen H , et al. MiR-106b-5p inhibits tumor necrosis factor-α-induced apoptosis by targeting phosphatase and tensin homolog deleted on chromosome 10 in vascular endothelial cells[J]. Chin Med J (Engl), 2016,129(12):1406-1412.
doi: 10.4103/0366-6999.183414 |
| [9] |
Ogata H, Goto S, Sato K , et al. KEGG: Kyoto Encyclopedia of Genes and Genomes[J]. Nucleic Acids Res, 1999,27(1):29-34.
doi: 10.1093/nar/27.1.29 |
| [10] |
Du J, Yuan Z, Ma Z , et al. KEGG-PATH: Kyoto encyclopedia of genes and genomes-based pathway analysis using a path analysis model[J]. Mol Biosyst, 2014,10(9):2441-2447.
doi: 10.1039/C4MB00287C |
| [11] |
Kanehisa M, Goto S, Sato Y , et al. KEGG for integration and interpretation of large-scale molecular data sets[J]. Nucleic Acids Res, 2012,40(Database issue):D109-114.
doi: 10.1093/nar/gkr988 |
| [12] |
Ross R . Atherosclerosis: an inflammatory disease[J]. N Engl J Med, 1999,340(2):115-126.
doi: 10.1056/NEJM199901143400207 |
| [13] | 任景怡, 许宁, 韩冠平 , 等. microRNAs 参与动脉粥样硬化疾病的发生[J]. 中国生物化学与分子生物学报, 2011,27(6):511-515. |
| [14] |
Zampetaki A, Willeit P, Drozdov I , et al. Profiling of circulating microRNAs: from single biomarkers to re-wired networks[J]. Cardiovasc Res, 2012,93(4):555-562.
doi: 10.1093/cvr/cvr266 |
| [15] |
Weber C, Noels H . Atherosclerosis: current pathogenesis and therapeutic options[J]. Nat Med, 2011,17(11):1410-1422.
doi: 10.1038/nm.2538 |
| [16] |
Zernecke A, Weber C . Chemokines in the vascular inflammatory response of atherosclerosis[J]. Cardiovasc Res, 2010,86(2):192-201.
doi: 10.1093/cvr/cvp391 |
| [17] |
Lutgens E, Daemen MJ . Transforming growth factor-β: a local or systemic mediator of plaque stability?[J]. Circ Res, 2001,89(10):853-855.
doi: 10.1161/res.89.10.853 |
| [18] |
Lutgens E, Gijbels M, Smook M , et al. Transforming growth factor-beta mediates balance between inflammation and fibrosis during plaque progression[J]. Arterioscler Thromb Vasc Biol, 2002,22(6):975-982.
doi: 10.1161/01.ATV.0000019729.39500.2F |
| [1] | 和静,房中则,杨颖,刘静,马文瑶,霍勇,高炜,武阳丰,谢高强. 血浆中脂质代谢分子与颈动脉粥样硬化斑块、传统心血管危险因素及膳食因素的关系[J]. 北京大学学报(医学版), 2024, 56(4): 722-728. |
| [2] | 任晓萌,李凯一,李春蕾. 基于转录组测序探索口腔扁平苔藓局部激素治疗敏感性相关分子特征[J]. 北京大学学报(医学版), 2024, 56(1): 32-38. |
| [3] | 李文根,古晓东,翁锐强,刘苏东,陈超. 血浆外泌体miR-34-5p和miR-142-3p在系统性硬化症中的表达及临床意义[J]. 北京大学学报(医学版), 2023, 55(6): 1022-1027. |
| [4] | 罗靓,李云,王红彦,相晓红,赵静,孙峰,张晓盈,贾汝琳,李春. 抗内皮细胞抗体检测在早期流产中的预测价值[J]. 北京大学学报(医学版), 2023, 55(6): 1039-1044. |
| [5] | 刘颖,霍然,徐慧敏,王筝,王涛,袁慧书. 磁共振血管壁成像评估颈动脉中重度狭窄患者斑块特征与脑血流灌注的相关性[J]. 北京大学学报(医学版), 2023, 55(4): 646-651. |
| [6] | 顾阳阳,谭晓辉,宋文鹏,方冬,宋卫东,袁亦铭,冯宁翰,关瑞礼. 4′-甲基醚金连木黄酮对棕榈酸诱导的大鼠阴茎海绵体内皮细胞功能障碍的影响[J]. 北京大学学报(医学版), 2022, 54(4): 599-604. |
| [7] | 申杰, 杨迪, 陈梦圆, 郭新彪. 长度和化学修饰在多壁碳纳米管诱导内皮细胞活化中的作用[J]. 北京大学学报(医学版), 2021, 53(3): 439-446. |
| [8] | 黄丽东,宫玮玉,董艳梅. 生物活性玻璃对人脐静脉血管内皮细胞增殖及成血管的作用[J]. 北京大学学报(医学版), 2021, 53(2): 371-377. |
| [9] | 刘滕飞,林涛,任利辉,李广平,彭建军. CMTM5基因与冠状动脉粥样硬化性心脏病的关联研究及机制探讨[J]. 北京大学学报(医学版), 2020, 52(6): 1082-1087. |
| [10] | 刘滕飞,林涛,任利辉,李广平,彭建军. CMTM5基因与冠心病患者支架内再狭窄发生风险[J]. 北京大学学报(医学版), 2020, 52(5): 856-862. |
| [11] | 徐涛,韩敬丽,姚伟娟. 雄激素剥夺治疗相关心血管疾病的机制与临床对策[J]. 北京大学学报(医学版), 2020, 52(4): 607-609. |
| [12] | 轩艳,蔡宇,王啸轩,石巧,邱立新,栾庆先. 牙龈卟啉单胞菌感染对载脂蛋白e基因敲除小鼠动脉粥样硬化的影响[J]. 北京大学学报(医学版), 2020, 52(4): 743-749. |
| [13] | 王晓,贺丹,李文婷,阿迪拉·斯依提,韩蕊,董颖. 144例维吾尔族与汉族女性子宫内膜癌微小RNA表达特点及临床意义[J]. 北京大学学报(医学版), 2020, 52(3): 570-577. |
| [14] | 任川,吴晓月,赵威,陶立元,刘萍,高炜. 心肺适能对动脉粥样硬化性心血管疾病高危患者的保护作用[J]. 北京大学学报(医学版), 2020, 52(1): 152-157. |
| [15] | 谢静,赵玉鸣,饶南荃,汪晓彤,方滕姣子,李晓霞,翟越,李静芝,葛立宏,王媛媛. 3种口腔颌面部来源的间充质干细胞成血管内皮分化潜能的比较研究[J]. 北京大学学报(医学版), 2019, 51(5): 900-906. |
|
||