北京大学学报(医学版) ›› 2020, Vol. 52 ›› Issue (6): 1082-1087. doi: 10.19723/j.issn.1671-167X.2020.06.015
CMTM5基因与冠状动脉粥样硬化性心脏病的关联研究及机制探讨
- 首都医科大学附属北京世纪坛医院心血管内科,北京 100034
Association between CMTM5 gene and coronary artery disease and the relative mechanism
Teng-fei LIU,Tao LIN,Li-hui REN,Guang-ping LI,Jian-jun PENG( )
)
- Department of Cardiology, Beijing Shijitan Hospital of Capital Medical University, Beijing 100034, China
摘要:
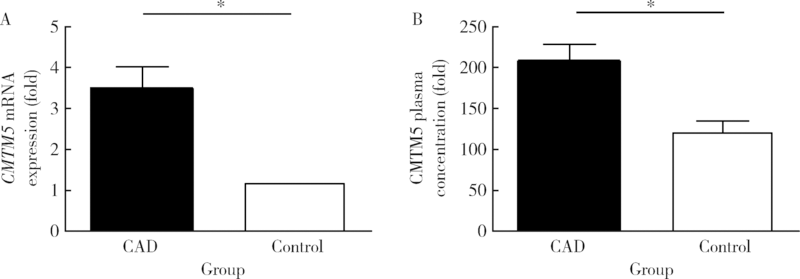
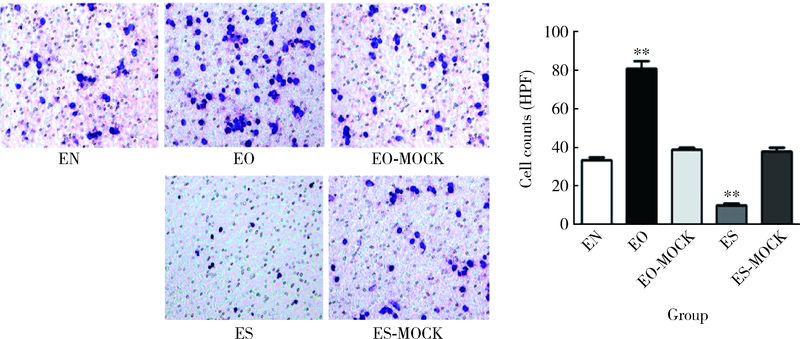
目的:探讨趋化素样因子超家族成员5(CKLF-like MARVEL transmembrane domain containing member 5,CMTM5)基因与冠状动脉粥样硬化性心脏病(简称冠心病)发生风险的相关性,及CMTM5基因表达变化对THP-1细胞黏附及迁移能力的影响。方法:采用病例对照研究法,入选700例首都医科大学附属北京世纪坛医院心血管内科的住院患者,采用冠状动脉造影法,将结果提示至少存在一支血管内径狭窄≥50%的患者诊断为冠心病。采用逆转录-聚合酶链反应法(reverse transcription-polymerase chain reaction,RT-PCR)测定CMTM5基因表达,酶联免疫吸附测定法(enzyme linked immunosorbent assay,ELISA)检测入选患者血浆CMTM5水平,Logistic回归方法分析CMTM5基因与冠心病发生风险的相关性。培养人血管内皮细胞(endothelial cells,ECs)及THP-1细胞,采用黏附实验及Transwells迁移实验评价CMTM5基因对THP-1趋化能力的影响。结果:冠心病组患者CMTM5基因mRNA表达量是对照组表达量的3.45倍,明显高于对照组(P<0.05)。冠心病组血浆CMTM5蛋白平均水平为(206.1±26.9) μg/L,明显高于对照组的(125.3±15.2) μg/L(P<0.05)。Logistic回归分析纳入年龄、性别、体重指数、吸烟、高血压、糖尿病、高脂血症等冠心病的易患因素和CMTM5基因,结果提示,CMTM5基因仍与冠心病的发生风险存在显著相关性(P<0.05)。黏附实验及Transwells实验结果均提示,过表达CMTM5 ECs组(EO组)中,THP-1细胞的黏附数量及迁移数量明显高于过表达CMTM5对照组(EO-MOCK组)、正常ECs组(EN组)、低表达CMTM5对照组(ES-MOCK组)和低表达CMTM5 ECs组(ES组), 相反,ES组中THP-1细胞黏附数量及迁移数量明显低于其他4组,差异均有统计学意义(P均<0.01)。结论:CMTM5基因与冠心病的发生发展密切相关,CMTM5基因过表达促进THP-1黏附及迁移能力,从而促进动脉粥样硬化和冠心病的发生发展。
中图分类号:
- R543.3
| [1] |
Hansson GK. Inflammation, atherosclerosis, and coronary artery disease[J]. N Engl J Med, 2005,352(16):1685-1695.
doi: 10.1056/NEJMra043430 pmid: 15843671 |
| [2] |
Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options[J]. Nat Med, 2011,17(11):1410-1422.
doi: 10.1038/nm.2538 pmid: 22064431 |
| [3] |
Aikawa M, Libby P. The vulnerable atherosclerotic plaque: pathogenesis and therapeutic approach[J]. Cardiovasc Pathol, 2004,13(3):125-138.
pmid: 15081469 |
| [4] |
Koenen RR, Weber C. Chemokines: established and novel targets in atherosclerosis[J]. EMBO Mol Med, 2011,3(12):713-725.
doi: 10.1002/emmm.201100183 |
| [5] |
Braunersreuther V, Mach F, Steffens S. The specific role of chemokines in atherosclerosis[J]. Thromb Haemost, 2007,97(5):714-721.
pmid: 17479181 |
| [6] |
Zernecke A, Shagdarsuren E, Weber C. Chemokines in atherosclerosis: an update[J]. Arterioscler Thromb Vasc Biol, 2008,28(11):1897-1908.
doi: 10.1161/ATVBAHA.107.161174 pmid: 18566299 |
| [7] |
Aukrust P, Halvorsen B, Yndestad A, et al. Chemokines and cardiovascular risk[J]. Arterioscler Thromb Vasc Biol, 2008,28(11):1909-1919.
doi: 10.1161/ATVBAHA.107.161240 pmid: 18669888 |
| [8] |
Li H, Guo X, Shao L, et al. CMTM5-v1, a four-transmembrane protein, presents a secreted form released via a vesicle-mediated secretory pathway[J]. BMB Rep, 2010,43(3):182-187.
pmid: 20356458 |
| [9] |
Voora D, Cyr D, Lucas J, et al. Aspirin exposure reveals novel genes associated with platelet function and cardiovascular events[J]. J Am Coll Cardiol, 2013,62(14):1267-1276.
doi: 10.1016/j.jacc.2013.05.073 pmid: 23831034 |
| [10] |
刘滕飞, 张婧薇, 陈夏欢, 等. CMTM5基因rs723840单核苷酸多态性与阿司匹林治疗下血小板高反应性的相关性研究[J]. 北京大学学报(医学版), 2015,47(6):905-909.
doi: 10.3969/j.issn.1671-167X.2015.06.003 |
| [11] |
Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation[J]. N Engl J Med, 2006,354(6):610-621.
doi: 10.1056/NEJMra052723 pmid: 16467548 |
| [12] |
Heydtmann M, Adams DH. Chemokines in the immunopathogenesis of hepatitis C infection[J]. Hepatology, 2009,49(2):676-688.
doi: 10.1002/hep.22763 pmid: 19177577 |
| [13] |
Golay J, Introna M. Chemokines and antagonists in non-Hodgkin’s lymphoma[J]. Expert Opin Ther Targets, 2008,12(5):621-635.
doi: 10.1517/14728222.12.5.621 pmid: 18410244 |
| [14] |
Tiemessen CT, Kuhn L. CC chemokines and protective immunity: insights gained from mother-to-child transmission of HIV[J]. Nat Immunol, 2007,8(3):219-222.
doi: 10.1038/ni0307-219 pmid: 17304227 |
| [15] |
Dhami H, Fritz CE, Gankin B, et al. The chemokine system and CCR5 antagonists: potential in HIV treatment and other novel therapies[J]. J Clin Pharm Ther, 2009,34(2):147-160.
doi: 10.1111/j.1365-2710.2008.00978.x pmid: 19250135 |
| [16] |
Lundberg GA, Kellin A, Samnegard A, et al. Severity of coronary artery stenosis is associated with a polymorphism in the CXCL16/SR-PSOX gene[J]. J Intern Med, 2005,257(5):415-422.
doi: 10.1111/j.1365-2796.2005.01469.x pmid: 15836657 |
| [17] |
Singh N, Rai H, Sinha N, et al. Association of V249I and T280M polymorphisms in the chemokine receptor CX3CR1 gene with early onset of coronary artery disease among North Indians[J]. Genet Test Mol Biomarkers, 2012,16(7):756-760.
doi: 10.1089/gtmb.2011.0256 pmid: 22731642 |
| [18] |
Cai W, Tao J, Zhang X, et al. Contribution of homeostatic chemokines CCL19 and CCL21 and their receptor CCR7 to coronary artery disease[J]. Arterioscler Thromb Vasc Biol, 2014,34(9):1933-1941.
pmid: 24990231 |
| [19] |
Zhang JW, Liu TF, Chen XH, et al. Validation of aspirin response-related transcripts in patients with coronary artery disease and preliminary investigation on CMTM5 function[J]. Gene, 2017,624:56-65.
doi: 10.1016/j.gene.2017.04.041 |
| [1] | 焦莶如, 龚潘, 牛悦, 徐兆, 周宗朴, 杨志仙. 以婴儿癫痫性痉挛综合征为表型的吡哆醇依赖性癫痫[J]. 北京大学学报(医学版), 2024, 56(5): 781-787. |
| [2] | 武志慧, 胡明智, 赵巧英, 吕凤凤, 张晶莹, 张伟, 王永福, 孙晓林, 王慧. miR-125b-5p修饰脐带间充质干细胞对系统性红斑狼疮的免疫调控机制[J]. 北京大学学报(医学版), 2024, 56(5): 860-867. |
| [3] | 和静,房中则,杨颖,刘静,马文瑶,霍勇,高炜,武阳丰,谢高强. 血浆中脂质代谢分子与颈动脉粥样硬化斑块、传统心血管危险因素及膳食因素的关系[J]. 北京大学学报(医学版), 2024, 56(4): 722-728. |
| [4] | 郭煌达,彭和香,王斯悦,侯天姣,李奕昕,章涵宇,王梦莹,武轶群,秦雪英,唐迅,李劲,陈大方,胡永华,吴涛. 短期大气颗粒物暴露和MTNR1B基因多态性对甘油三酯-葡萄糖指数影响的家系研究[J]. 北京大学学报(医学版), 2024, 56(3): 375-383. |
| [5] | 侯天姣,周治波,王竹青,王梦莹,王斯悦,彭和香,郭煌达,李奕昕,章涵宇,秦雪英,武轶群,郑鸿尘,李静,吴涛,朱洪平. 转化生长因子β信号通路与非综合征型唇腭裂发病风险的基因-基因及基因-环境交互作用[J]. 北京大学学报(医学版), 2024, 56(3): 384-389. |
| [6] | 王鹏,杨子瑶,王萌,王巍,李爱芝. 2例罕见RhD变异型RHD*DEL37的分子生物学分析[J]. 北京大学学报(医学版), 2024, 56(2): 352-356. |
| [7] | 刘欢锐,彭祥,李森林,苟欣. 基于HER-2相关基因构建风险模型用于膀胱癌生存预后评估[J]. 北京大学学报(医学版), 2023, 55(5): 793-801. |
| [8] | 金银姬,孙琳,赵金霞,刘湘源. 血清IgA型抗鼠科肉瘤病毒癌基因同源物B1抗体在类风湿关节炎中的意义[J]. 北京大学学报(医学版), 2023, 55(4): 631-635. |
| [9] | 刘颖,霍然,徐慧敏,王筝,王涛,袁慧书. 磁共振血管壁成像评估颈动脉中重度狭窄患者斑块特征与脑血流灌注的相关性[J]. 北京大学学报(医学版), 2023, 55(4): 646-651. |
| [10] | 谢尚,蔡志刚,单小峰. 全外显子测序及相关指标在口腔鳞状细胞癌精准治疗中的应用价值[J]. 北京大学学报(医学版), 2023, 55(4): 697-701. |
| [11] | 许媛媛,孙志琳,张秀莲,刘子莲,刘维,关欣. 卡马西平致HLA-A * 3101基因阳性中国汉族人发生Stevens-Johnson综合征1例[J]. 北京大学学报(医学版), 2023, 55(4): 755-757. |
| [12] | 史佳琪,马莺,张奕,陈章健,贾光. 纳米二氧化钛颗粒对人肝癌细胞HepG2中circRNA表达谱的影响[J]. 北京大学学报(医学版), 2023, 55(3): 392-399. |
| [13] | 王雪珩,王斯悦,彭和香,范梦,郭煌达,侯天姣,王梦莹,武轶群,秦雪英,唐迅,李劲,陈大方,胡永华,吴涛. 基因-环境交互作用对动脉僵硬度影响的家系研究[J]. 北京大学学报(医学版), 2023, 55(3): 400-407. |
| [14] | 时云飞,王豪杰,刘卫平,米岚,龙孟平,刘雁飞,赖玉梅,周立新,刁新婷,李向红. 血管免疫母细胞性T细胞淋巴瘤临床与分子病理学特征分析[J]. 北京大学学报(医学版), 2023, 55(3): 521-529. |
| [15] | 王微,李鑫,柳萍,董颖. 荧光原位杂交检测MDM2和DDIT3基因信号改变在诊断脂肪肉瘤中的价值[J]. 北京大学学报(医学版), 2023, 55(2): 228-233. |
|
||