北京大学学报(医学版) ›› 2023, Vol. 55 ›› Issue (3): 521-529. doi: 10.19723/j.issn.1671-167X.2023.03.019
血管免疫母细胞性T细胞淋巴瘤临床与分子病理学特征分析
时云飞1,王豪杰2,刘卫平3,米岚3,龙孟平1,刘雁飞3,赖玉梅1,周立新1,刁新婷1,李向红1,*( )
)
- 1. 北京大学肿瘤医院暨北京市肿瘤防治研究所病理科,恶性肿瘤发病机制及转化研究教育部重点实验室,北京 100142
2. 北京大学肿瘤医院暨北京市肿瘤防治研究所中心实验室,恶性肿瘤发病机制及转化研究教育部重点实验室,北京 100142
3. 北京大学肿瘤医院暨北京市肿瘤防治研究所淋巴瘤科,恶性肿瘤发病机制及转化研究教育部重点实验室,北京 100142
Analysis of clinicopathological and molecular abnormalities of angioimmunoblastic T-cell lymphoma
Yun-fei SHI1,Hao-jie WANG2,Wei-ping LIU3,Lan MI3,Meng-ping LONG1,Yan-fei LIU3,Yu-mei LAI1,Li-xin ZHOU1,Xin-ting DIAO1,Xiang-hong LI1,*( )
)
- 1. Department of Pathology, Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education), Peking University Cancer Hospital & Institute, Beijing 100142, China
2. Central Laboratory, Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education), Peking University Cancer Hospital & Institute, Beijing 100142, China
3. Department of Lymphoma, Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education), Peking University Cancer Hospital & Institute, Beijing 100142, China
摘要:
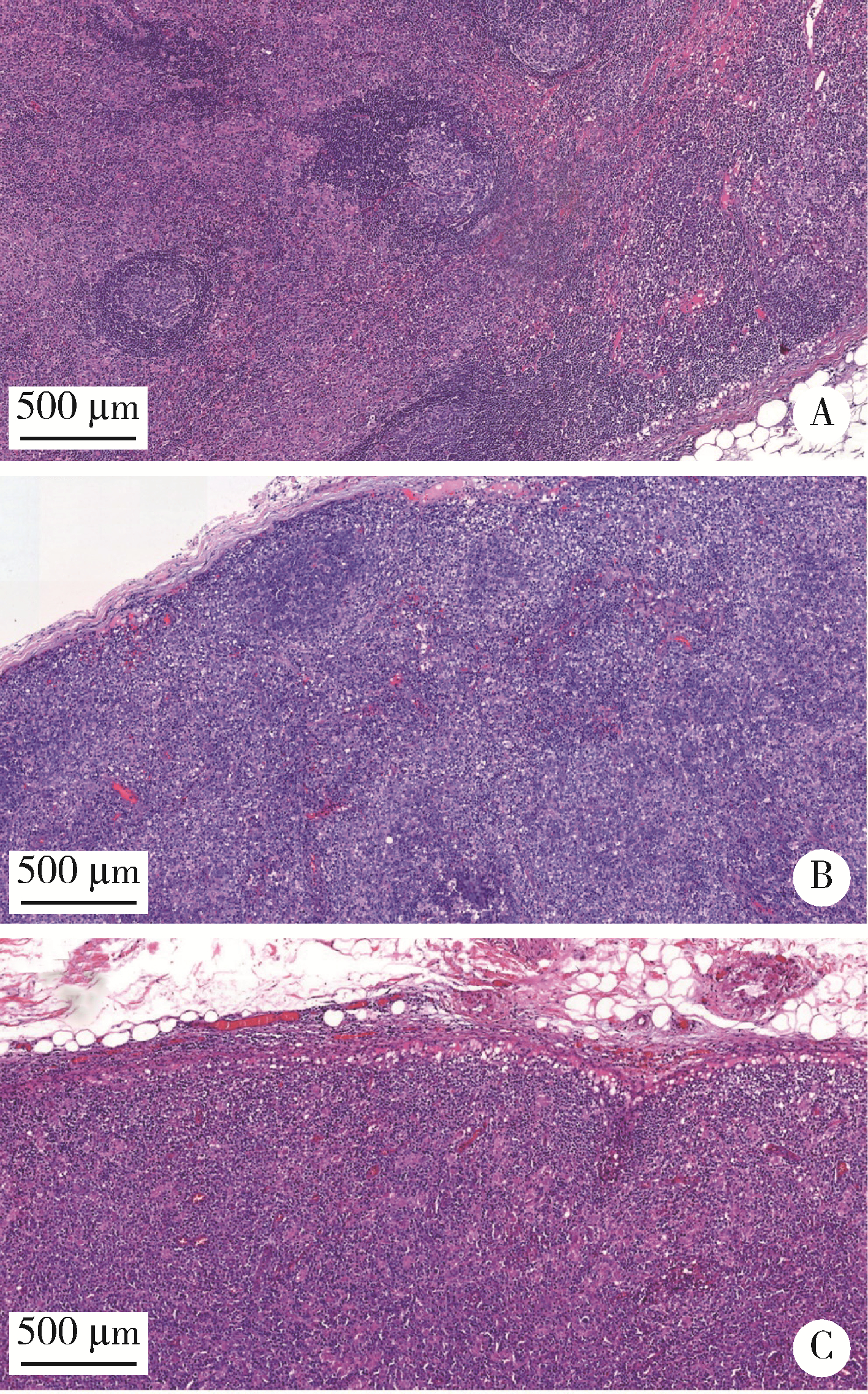
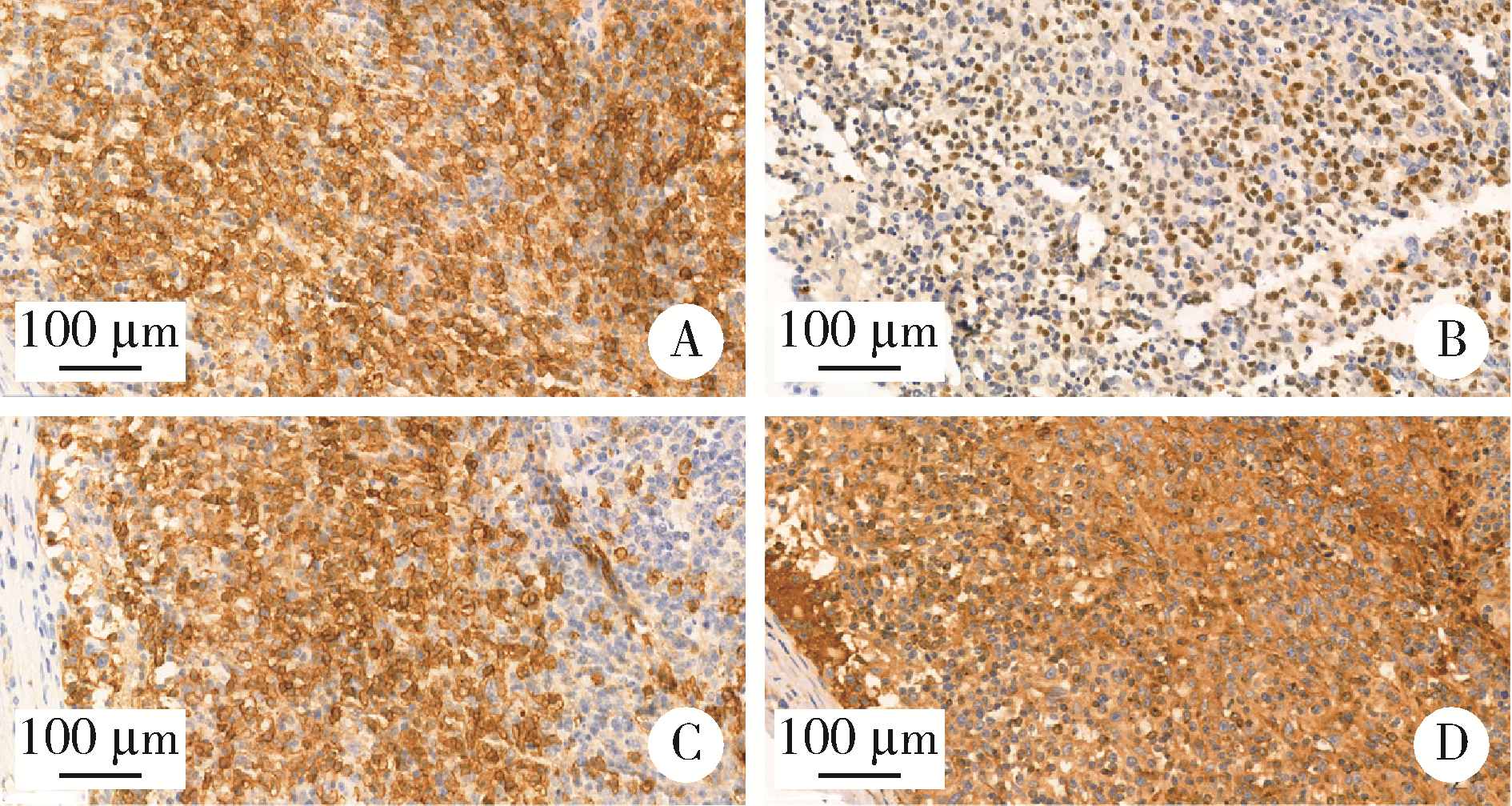
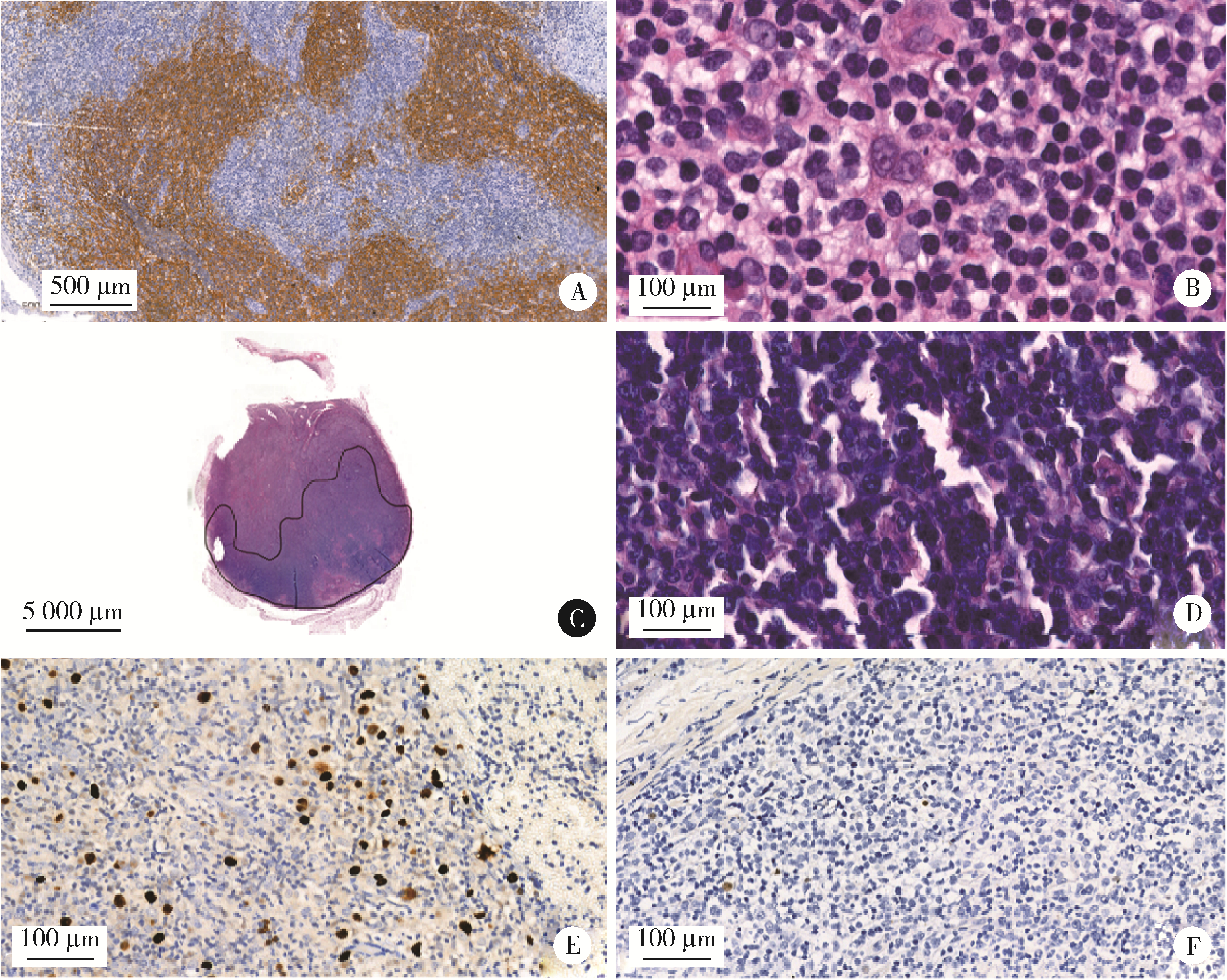
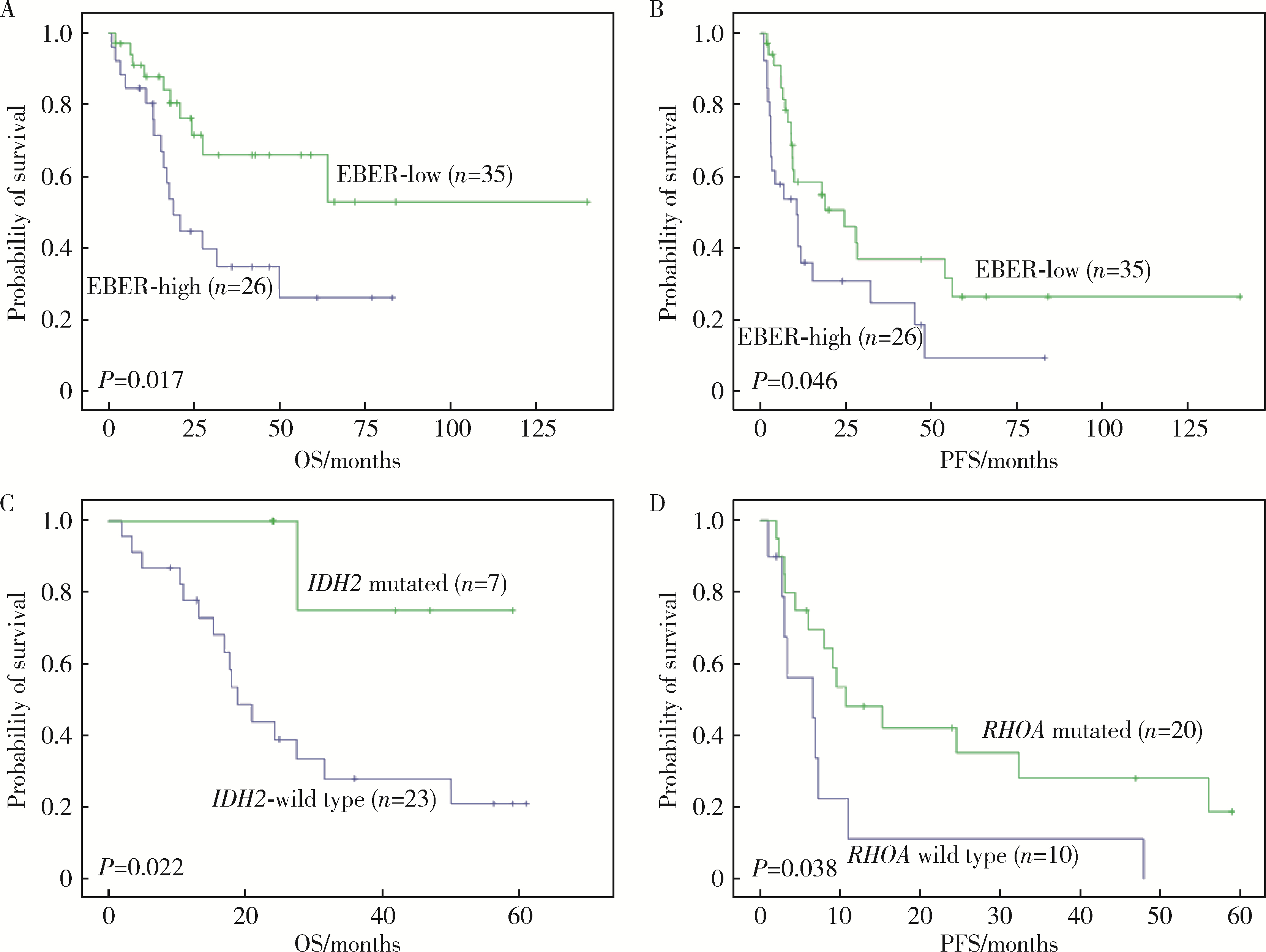
目的: 分析血管免疫母细胞性T细胞淋巴瘤(angioimmunoblastic T-cell lymphoma,AITL)病理学特征、基因改变及预后的影响因素。方法: 选择北京大学肿瘤医院病理科2007年6月至2021年11月明确诊断且有完整随访信息的AITL患者病例资料进行回顾性分析,对病例进行形态学分型[Ⅰ型:淋巴结反应性增生(lymphoid tissue reactive hyperplasia,LRH)样;Ⅱ型:边缘区淋巴瘤(marginal zone lymphoma,MZL)样;Ⅲ型:外周T细胞淋巴瘤非特指型(peripheral T-cell lymphoma, not specified,PTCL-NOS)样]。结合免疫组化染色评估肿瘤有无滤泡辅助T细胞(follicular helper T cell,TFH)表型,生发中心(germinal center,GC)外滤泡树突细胞(follicular dendritic cells,FDC)增生,Hodgkin和Reed-Sternberg (HRS)样细胞及大B(细胞淋巴瘤)转化;计数每个高倍视野(high power field,HPF)Epstein-Barr病毒(Epstein-Barr virus,EBV)阳性细胞含量;按需要完善T细胞受体(T-cell receptor,TCR) / 免疫球蛋白(immunoglobulin,IG)基因重排检测和靶向外显子二代测序(targeted exome sequencing,TES)检测。结果: 共收集患者61例,形态分型Ⅰ型7例(11.4%)、Ⅱ型31例(50.8%)、Ⅲ型23例(37.8%),51例(83.6%)具有明确TFH表型,有不同程度GC外FDC网架增生(中位20.0%),14例(23.0%)有HRS样细胞,7例(11.5%)有大B转化。42.6%(26/61)的病例属于EBV高含量(>10个/HPF)。TCR/IG重排分析57.9%TCR+/IG-(11/19),26.3%TCR+/IG+(5/19),10.5%TCR-/IG-(2/19),5.3%TCR-/IG+(1/19)。TES检测RHOA突变66.7%(20/30),IDH2突变23.3%(7/30),TET2突变80.0%(24/30),DNMT3A突变33.3%(10/30)。TES检测30例患者分为(1)IDH2和RHOA共突变组(7例):Ⅱ型6例,Ⅲ型1例,具典型TFH表型,未见HRS细胞和大B转化;(2)单RHOA突变组(13例):Ⅰ型1例,Ⅱ型6例,Ⅲ型6例,不具典型TFH表型5例,伴HRS细胞6例,伴大B转化2例,TCR-/IG- 1例, TCR-/IG+ 1例,TCR+/IG+ 1例;(3)仅TET2和/或DNMT3A突变组(7例):Ⅱ型3例,Ⅲ型4例,均具典型TFH表型,伴HRS细胞2例,伴大B转化2例;(4)无突变组(3例),均为Ⅱ型,TFH表型典型,FDC网架增生,TCR-/IG- 1例。单因素生存分析证实EBV阳性细胞含量高为总生存和无进展生存的不良预后因素(P=0.017及P=0.046)。结论: AITL组织病理学难以诊断主要见于形态Ⅰ型,伴HRS样细胞,大B转化;此时TCR/IGH重排检测对诊断有帮助但价值有限;增加TES检测RHOA、IDH2、TET2、DNMT3A等基因突变可有效辅助AITL疑难病例的组织病理学诊断。肿瘤组织中EBV阳性细胞含量升高提示预后不良。
中图分类号:
- R732.2
| 1 |
Swerdlow SH , Campo E , Pileri SA , et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms[J]. Blood, 2016, 127 (20): 2375- 2390.
doi: 10.1182/blood-2016-01-643569 |
| 2 |
Laurent C , Baron M , Amara N , et al. Impact of expert pathologic review of lymphoma diagnosis: Study of patients from the French lymphopath network[J]. J Clin Oncol, 2017, 35 (18): 2008- 2017.
doi: 10.1200/JCO.2016.71.2083 |
| 3 | van Krieken JHJM , Langerak AW , Macintyre EA , et al. Improved reliability of lymphoma diagnostics via PCR-based clonality testing: Report of the BIOMED-2 concerted action BHM4-CT98-3936[J]. Leukemia, 2006, 21 (2): 201- 206. |
| 4 |
Langerak AW , Groenen PJTA , Brüggemann M , et al. Euro-Clonality/BIOMED-2 guidelines for interpretation and reporting of Ig/TCR clonality testing in suspected lymphoproliferations[J]. Leukemia, 2012, 26 (10): 2159- 2171.
doi: 10.1038/leu.2012.246 |
| 5 |
Cortes JR , Palomero T . The curious origins of angioimmunoblastic T-cell lymphoma[J]. Curr Opin Hematol, 2016, 23 (4): 434- 443.
doi: 10.1097/MOH.0000000000000261 |
| 6 | 郭艳敏, 刘雪霏, 焦莉娟, 等. 血管免疫母细胞性T细胞淋巴瘤组织学分级与预后分析[J]. 中华病理学杂志, 2019, 48 (10): 784- 790. |
| 7 |
Zhang C , Mi L , Wu M , et al. Angioimmunoblastic T-cell lymphoma: Treatment strategies and prognostic factors from a retrospective multicenter study in China[J]. Leuk Lymphoma, 2022, 63 (5): 1152- 1159.
doi: 10.1080/10428194.2021.2015586 |
| 8 |
Hsu YT , Wang YC , Chen RY , et al. Angioimmunoblastic T-cell lymphoma in Taiwan reveals worse progression-free survival for RHOA G17V mutated subtype[J]. Leuk Lymphoma, 2020, 61 (5): 1108- 1118.
doi: 10.1080/10428194.2019.1702179 |
| 9 | 李婷婷, 罗璐婷, 陈溢, 等. 84例血管免疫母细胞性T细胞淋巴瘤的临床特征及预后: 单中心分析[J]. 中华血液学杂志, 2020, 41 (11): 915- 920. |
| 10 |
Steinhilber J , Mederake M , Bonzheim I , et al. The pathological features of angioimmunoblastic T-cell lymphomas with IDH2(R172) mutations[J]. Mod Pathol, 2019, 32 (8): 1123- 1134.
doi: 10.1038/s41379-019-0254-4 |
| 11 |
Hsi ED , Said J , Macon WR , et al. Diagnostic accuracy of a defined immunophenotypic and molecular genetic approach for peripheral T/NK-cell lymphomas. A North American PTCL study group project[J]. Am J Surg Pathol, 2014, 38 (6): 768- 775.
doi: 10.1097/PAS.0000000000000188 |
| 12 |
Heavican TB , Bouska A , Yu J , et al. Genetic drivers of oncogenic pathways in molecular subgroups of peripheral T-cell lymphoma[J]. Blood, 2019, 133 (15): 1664- 1676.
doi: 10.1182/blood-2018-09-872549 |
| 13 |
Schwartz FH , Cai Q , Fellmann E , et al. TET2 mutations in B cells of patients affected by angioimmunoblastic T-cell lymphoma[J]. J Pathol, 2017, 242 (2): 129- 133.
doi: 10.1002/path.4898 |
| 14 |
Palomero T , Couronne L , Khiabanian H , et al. Recurrent mutations in epigenetic regulators, RHOA and FYN kinase in peripheral T cell lymphomas[J]. Nature Genetics, 2014, 46 (2): 166.
doi: 10.1038/ng.2873 |
| 15 |
Yoo HY , Sung MK , Lee SH , et al. A recurrent inactivating mutation in RHOA GTPase in angioimmunoblastic T cell lymphoma[J]. Nat Genet, 2014, 46 (4): 371- 375.
doi: 10.1038/ng.2916 |
| 16 |
Fukumoto K , Nguyen TB , Chiba S , et al. Review of the biologic and clinical significance of genetic mutations in angioimmunoblastic T-cell lymphoma[J]. Cancer Sci, 2018, 109 (3): 490- 496.
doi: 10.1111/cas.13393 |
| 17 |
Chiba S , Sakata-Yanagimoto M . Advances in understanding of angioimmunoblastic T-cell lymphoma[J]. Leukemia, 2020, 34 (10): 2592- 2606.
doi: 10.1038/s41375-020-0990-y |
| 18 | 中国临床肿瘤学会肿瘤标志物专家委员会, 中国肿瘤驱动基因分析联盟. 二代测序技术在肿瘤精准医学诊断中的应用专家共识[J]. 中华医学杂志, 2018, 98 (26): 2057- 2065. |
| 19 | 中国抗癌协会血液肿瘤专业委员会, 中华医学会血液学分会, 中华医学会病理学分会. 二代测序技术在血液肿瘤中的应用中国专家共识(2018年版)[J]. 中华血液学杂志, 2018, 39 (11): 881- 886. |
| 20 |
Ondrejka SL , Grzywacz B , Bodo J , et al. Angioimmunoblastic T-cell lymphomas with the RHOA p.Gly17Val mutation have classic clinical and pathologic features[J]. Am J Surg Pathol, 2016, 40 (3): 335- 341.
doi: 10.1097/PAS.0000000000000555 |
| 21 | 张晨, 王小沛, 郑文, 等. 血管免疫母细胞性T细胞淋巴瘤42例临床分析[J]. 中华医学杂志, 2013, 93 (46): 3671- 3674. |
| 22 | Eladl AE , Shimada K , Suzuki Y , et al. EBV status has prognostic implication among young patients with angioimmunoblastic T-cell lymphoma[J]. Cancer Med, 2020, 9 (2): 678- 688. |
| 23 | 王芳, 张瑰红, 丁凯阳, 等. EBER、PTEN和VEGF在血管免疫母T细胞淋巴瘤中的表达及其临床病理学意义[J]. 中国实验血液学杂志, 2015, 23 (3): 663- 668. |
| 24 | Liang JH , Lu L , Zhu HY , et al. The prognostic role of circulating Epstein-Barr Virus DNA copy number in angioimmunoblastic T-cell lymphoma treated with dose-adjusted EPOCH[J]. Cancer Res Treat, 2019, 51 (1): 150- 157. |
| 25 | Kim TY , Min GJ , Jeon YW , et al. Impact of Epstein-Barr virus on peripheral T-cell lymphoma not otherwise specified and angioimmunoblastic T-cell lymphoma[J]. Front Oncol, 2021, 11, 797028. |
| [1] | 李挺. 建设当代临床病理学科[J]. 北京大学学报(医学版), 2023, 55(2): 197-200. |
| [2] | 朱晓娟,张虹,张爽,李东,李鑫,徐玲,李挺. 人表皮生长因子受体2低表达乳腺癌的临床病理学特征及预后[J]. 北京大学学报(医学版), 2023, 55(2): 243-253. |
| [3] | 赖玉梅,李忠武,李欢,吴艳,时云飞,周立新,楼雨彤,崔传亮. 68例肛管直肠黏膜黑色素瘤临床病理特征及预后[J]. 北京大学学报(医学版), 2023, 55(2): 262-269. |
| [4] | 刘菊梅,梁丽,张继新,戎龙,张梓怡,吴悠,赵旭东,李挺. 411例早期胃癌及癌前病变内镜黏膜下剥离术标本的病理学评估[J]. 北京大学学报(医学版), 2023, 55(2): 299-307. |
| [5] | 农琳,王微,梁丽,李东,李鑫,李挺. 母细胞性浆样树突细胞肿瘤13例临床病理学特征[J]. 北京大学学报(医学版), 2023, 55(2): 308-314. |
| [6] | 熊焰,张波,聂立功,吴世凯,赵虎,李东,邸吉廷. 胸部SMARCA4缺失性未分化肿瘤的病理诊断与联合免疫检测点抑制剂治疗[J]. 北京大学学报(医学版), 2023, 55(2): 351-356. |
| [7] | 宁博涵,张青霞,杨慧,董颖. 伴间质细胞增生、玻璃样变性及索状结构的子宫内膜样腺癌1例[J]. 北京大学学报(医学版), 2023, 55(2): 366-369. |
| [8] | 周桥. 肿瘤病理学研究的进展和展望[J]. 北京大学学报(医学版), 2023, 55(2): 201-209. |
| [9] | 沈棋,刘亿骁,何群. 肾黏液样小管状和梭形细胞癌的临床病理特点及预后[J]. 北京大学学报(医学版), 2023, 55(2): 276-282. |
| [10] | 侯卫华,宋书杰,石中月,金木兰. 幽门螺杆菌阴性早期胃癌的临床病理特征[J]. 北京大学学报(医学版), 2023, 55(2): 292-298. |
| [11] | 哈雪梅,姚永正,孙莉华,辛春杨,熊焰. 实性肺胎盘样变形1例及文献复习[J]. 北京大学学报(医学版), 2023, 55(2): 357-361. |
| [12] | 宿骞,彭歆,周传香,俞光岩. 369例口腔颌面部非霍奇金淋巴瘤的临床病理特点及预后[J]. 北京大学学报(医学版), 2023, 55(1): 13-21. |
| [13] | 张铨,宋海峰,马冰磊,张喆楠,周朝晖,李傲林,刘军,梁磊,朱时雨,张骞. 术前预后营养指数可作为预测非转移性肾细胞癌预后的指标[J]. 北京大学学报(医学版), 2023, 55(1): 149-155. |
| [14] | 邢晓燕,张筠肖,朱冯赟智,王一帆,周新尧,李玉慧. 皮肌炎合并巨噬细胞活化综合征5例[J]. 北京大学学报(医学版), 2022, 54(6): 1214-1218. |
| [15] | 程晓静,蒋栋,张连海,王江华,李雅真,翟佳慧,闫宝琪,张露露,谢兴旺,李子禹,季加孚. KRAS G12V特异性T细胞受体治疗恶性肿瘤的临床前研究[J]. 北京大学学报(医学版), 2022, 54(5): 884-895. |
|
||