北京大学学报(医学版) ›› 2023, Vol. 55 ›› Issue (1): 149-155. doi: 10.19723/j.issn.1671-167X.2023.01.023
术前预后营养指数可作为预测非转移性肾细胞癌预后的指标
张铨,宋海峰,马冰磊,张喆楠,周朝晖,李傲林,刘军,梁磊,朱时雨,张骞*( )
)
- 北京大学第一医院泌尿外科,北京大学泌尿外科研究所,国家泌尿、男性生殖系肿瘤研究中心,北京 100034
Pre-operative prognostic nutritional index as a predictive factor for prognosis in non-metastatic renal cell carcinoma treated with surgery
Quan ZHANG,Hai-feng SONG,Bing-lei MA,Zhe-nan ZHANG,Chao-hui ZHOU,Ao-lin LI,Jun LIU,Lei LIANG,Shi-yu ZHU,Qian ZHANG*( )
)
- Department of Urology, Peking University First Hospital; Institute of Urology, Peking University; National Urological Cancer Center, Beijing 100034, China
摘要:
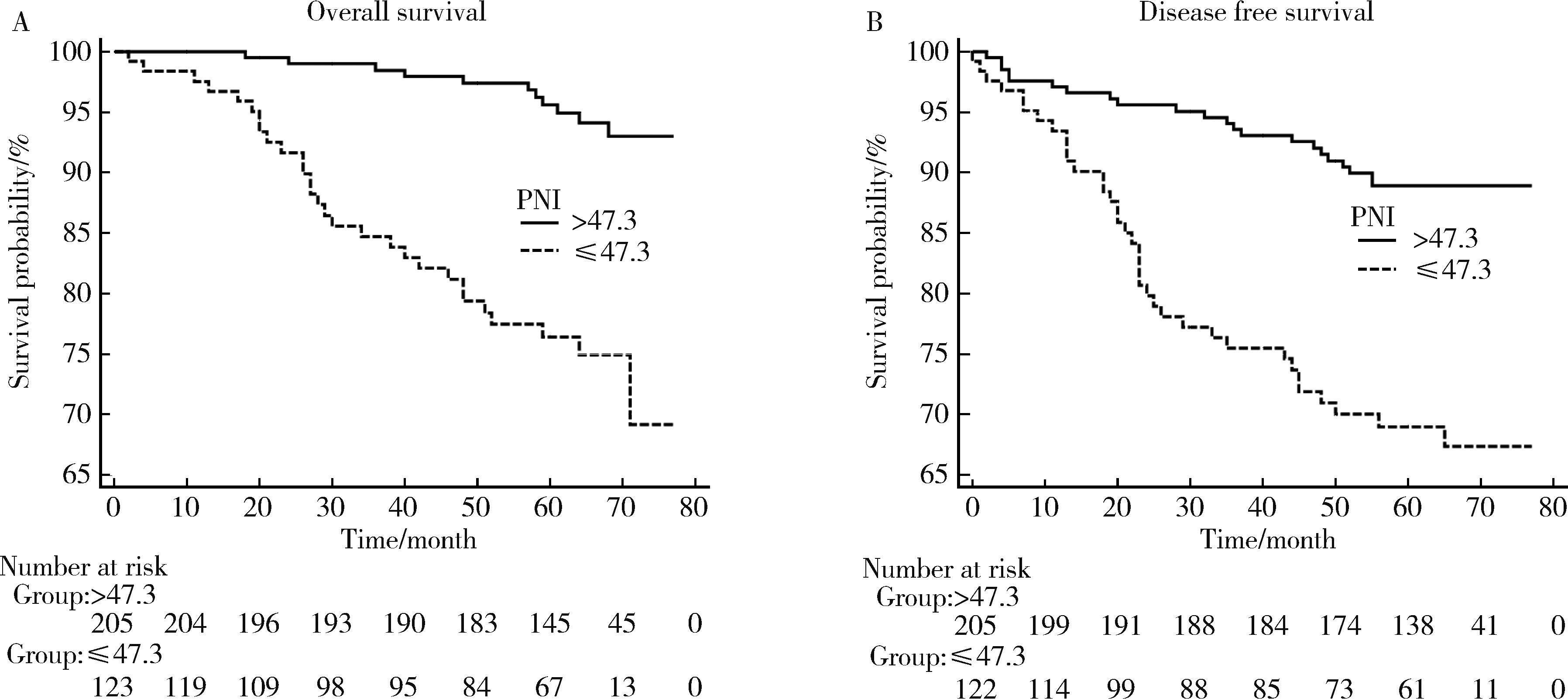
目的: 探讨预后营养指数(prognostic nutrition index, PNI)对非转移性肾细胞癌术后患者预后的意义,并将PNI与中性粒细胞与淋巴细胞比值(neutrophil to lymphocyte ratio, NLR)、血小板与淋巴细胞比值(platelet to lymphocyte ratio, PLR)、全身免疫炎症指数(systemic immune inflammation index, SII)等血液学指标进行比较。方法: 回顾性收集2010年1月至2012年12月于北京大学第一医院泌尿外科接受手术治疗的328例非转移性肾细胞癌患者的临床病理资料。采用受试者工作特征(receiver operating characteristic, ROC)曲线分析各个血液学指标的敏感度、特异度,根据最大约登指数(Youden index)确定其最佳截断值。采用Kaplan-Meier法绘制术后生存曲线,Cox回归模型分析PNI与总生存期(overall survival, OS)、无病生存期(disease-free survival, DFS)的相关性。结果: 根据ROC曲线最大约登指数得出PNI最佳截断值为47.3。低水平的PNI与患者高龄、低体重指数、更高的肿瘤病理T分期相关(P < 0.05)。Kaplan-Meier单因素分析显示,较低的PNI与较差的OS和DFS均显著相关(P < 0.05),此外,高龄、低体重指数、肿瘤坏死、更高的肿瘤病理T分期及Fuhrman分级均与较差的OS显著相关(P < 0.05)。Cox多因素分析结果表明,4种血液学指标中,只有PNI不论作为连续变量(HR=0.9,95%CI=0.828~0.978,P=0.013)还是分类变量(HR=2.397,95%CI=1.061~5.418,P=0.036)都是影响OS的独立因素。结论: 非转移性肾细胞癌患者术前低PNI是术后高病理T分期的重要预测指标,同时也是术后OS、DFS不佳的独立危险因素。PNI作为预测肾细胞癌患者的预后指标优于其他血清学指标。
中图分类号:
- R737.11
| 1 |
Siegel RL , Miller KD , Jemal A . Cancer statistics, 2015[J]. CA Cancer J Clin, 2015, 65 (1): 5- 29.
doi: 10.3322/caac.21254 |
| 2 |
Janzen NK , Kim HL , Figlin RA , et al. Surveillance after radical or partial nephrectomy for localized renal cell carcinoma and management of recurrent disease[J]. Urol Clin North Am, 2003, 30 (4): 843- 852.
doi: 10.1016/S0094-0143(03)00056-9 |
| 3 |
Chrom P , Stec R , Bodnar L , et al. Incorporating neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in place of neutrophil count and platelet count improves prognostic accuracy of the International Metastatic Renal Cell Carcinoma Database Consortium model[J]. Cancer Res Treat, 2018, 50 (1): 103- 110.
doi: 10.4143/crt.2017.033 |
| 4 | Bazzi WM , Tin AL , Sjoberg DD , et al. The prognostic utility of preoperative neutrophil-to-lymphocyte ratio in localized clear cell renal cell carcinoma[J]. Can J Urol, 2016, 23 (1): 8151- 8154. |
| 5 | Hu K , Lou L , Ye J , et al. Prognostic role of the neutrophil-lymphocyte ratio in renal cell carcinoma: A meta-analysis[J]. BMJ Open, 2015, 5 (4): 6404- 6415. |
| 6 |
Jagdev SP , Gregory W , Vasudev NS , et al. Improving the accuracy of pre-operative survival prediction in renal cell carcinoma with C-reactive protein[J]. Br J Cancer, 2010, 103 (11): 1649- 1656.
doi: 10.1038/sj.bjc.6605973 |
| 7 | Onodera T , Goseki N , Kosaki G . Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients[J]. Nihon Geka Gakkai Zasshi, 1984, 85 (9): 1001- 1005. |
| 8 |
Borda A , Borda F , Vila J , et al. Predictive pre-treatment value of the prognostic nutritional index on survival in gastric carcinoma[J]. An Sist Sanit Navar, 2016, 39 (2): 227- 235.
doi: 10.23938/ASSN.0271 |
| 9 |
Nozoe T , Ninomiya M , Maeda T , et al. Prognostic nutritional index: A tool to predict the biological aggressiveness of gastric carcinoma[J]. Surg Today, 2010, 40 (5): 440- 443.
doi: 10.1007/s00595-009-4065-y |
| 10 |
Nozoe T , Kohno M , Iguchi T , et al. The prognostic nutritional index can be a prognostic indicator in colorectal carcinoma[J]. Surg Today, 2012, 42 (6): 532- 535.
doi: 10.1007/s00595-011-0061-0 |
| 11 |
Peng D , Gong YQ , Hao H , et al. Preoperative prognostic nutritional index is a significant predictor of survival with bladder cancer after radical cystectomy: A retrospective study[J]. BMC Cancer, 2017, 17 (1): 391- 399.
doi: 10.1186/s12885-017-3372-8 |
| 12 |
Xue W , Tan P , Xu H , et al. Impact of the preoperative prognostic nutritional index on survival outcomes in upper tract urothelial carcinomas[J]. Cancer Med, 2019, 8 (6): 2971- 2978.
doi: 10.1002/cam4.2161 |
| 13 | Cui J , Chen S , Bo Q , et al. Preoperative prognostic nutritional index and nomogram predicting recurrence-free survival in patients with primary non-muscle-invasive bladder cancer without carcinoma in situ[J]. Onco Targets Ther, 2017, 10 (1): 5541- 5550. |
| 14 |
Kang M , Chang CT , Sung HH , et al. Prognostic significance of pre- to postoperative dynamics of the prognostic nutritional index for patients with renal cell carcinoma who underwent radical nephrectomy[J]. Ann Surg Oncol, 2017, 24 (13): 4067- 4075.
doi: 10.1245/s10434-017-6065-2 |
| 15 |
Jeon HG , Choi DK , Sung HH , et al. Preoperative prognostic nutritional index is a significant predictor of survival in renal cell carcinoma patients undergoing nephrectomy[J]. Ann Surg Oncol, 2016, 23 (1): 321- 327.
doi: 10.1245/s10434-015-4614-0 |
| 16 |
Kang HW , Seo SP , Kim WT , et al. Low preoperative serum cholesterol level is associated with aggressive pathologic features and poor cancer-specific survival in patients with surgically treated renal cell carcinoma[J]. Int J Clin Oncol, 2018, 23 (1): 142- 150.
doi: 10.1007/s10147-017-1172-4 |
| 17 |
de Martino M , Leitner CV , Seemann C , et al. Preoperative serum cholesterol is an independent prognostic factor for patients with renal cell carcinoma (RCC)[J]. BJU Int, 2015, 115 (3): 397- 404.
doi: 10.1111/bju.12767 |
| 18 |
Chen Z , Shao Y , Wang K , et al. Prognostic role of pretreatment serum albumin in renal cell carcinoma: A systematic review and meta-analysis[J]. Onco Targets Ther, 2016, 9, 6701- 6710.
doi: 10.2147/OTT.S108469 |
| 19 | Byun SS , Hwang EC , Kang SH , et al. Prognostic significance of preoperative neutrophil-to-lymphocyte ratio in nonmetastatic renal cell carcinoma: A large, multicenter cohort analysis[J]. Biomed Res Int, 2016, 2016, 4148- 4156. |
| 20 | Grimes N , Hannan C , Tyson M , et al. The role of neutrophil-lymphocyte ratio as a prognostic indicator in patients undergoing nephrectomy for renal cell carcinoma[J]. Can Urol Assoc J, 2018, 12 (7): E345- E348. |
| 21 | Zhou W , Zhang GL . C-reactive protein to albumin ratio predicts the outcome in renal cell carcinoma: A meta-analysis[J]. PLoS One, 2019, 14 (10): 4266- 4277. |
| 22 |
Wang X , Su S , Guo Y . The clinical use of the platelet to lymphocyte ratio and lymphocyte to monocyte ratio as prognostic factors in renal cell carcinoma: A systematic review and meta-analysis[J]. Oncotarget, 2017, 8 (48): 84506- 84514.
doi: 10.18632/oncotarget.21108 |
| 23 |
Jiang N , Deng JY , Ding XW , et al. Prognostic nutritional index predicts postoperative complications and long-term outcomes of gastric cancer[J]. World J Gastroenterol, 2014, 20 (30): 10537- 10544.
doi: 10.3748/wjg.v20.i30.10537 |
| 24 |
Zheng Y , Bao L , Wang W , et al. Prognostic impact of the controlling nutritional status score following curative nephrectomy for patients with renal cell carcinoma[J]. Medicine (Baltimore), 2018, 97 (49): e13409.
doi: 10.1097/MD.0000000000013409 |
| 25 |
Kanda M , Mizuno A , Tanaka C , et al. Nutritional predictors for postoperative short-term and long-term outcomes of patients with gastric cancer[J]. Medicine (Baltimore), 2016, 95 (24): e3781.
doi: 10.1097/MD.0000000000003781 |
| 26 |
Song Y , Yang Y , Gao P , et al. The preoperative neutrophil to lymphocyte ratio is a superior indicator of prognosis compared with other inflammatory biomarkers in resectable colorectal cancer[J]. BMC Cancer, 2017, 17 (1): 391- 399.
doi: 10.1186/s12885-017-3372-8 |
| 27 |
Hu H , Yao X , Xie X , et al. Prognostic value of preoperative NLR, dNLR, PLR and CRP in surgical renal cell carcinoma patients[J]. World J Urol, 2017, 35 (2): 261- 270.
doi: 10.1007/s00345-016-1864-9 |
| 28 | Tsujino T , Komura K , Hashimoto T , et al. C-reactive protein-albumin ratio as a prognostic factor in renal cell carcinoma: A data from multi-institutional study in Japan[J]. Urol Oncol, 2019, 37 (11): 812.e1- 812.e8. |
| 29 |
Lien YC , Hsieh CC , Wu YC , et al. Preoperative serum albumin level is a prognostic indicator for adenocarcinoma of the gastric cardia[J]. J Gastrointest Surg, 2004, 8 (8): 1041- 1048.
doi: 10.1016/j.gassur.2004.09.033 |
| 30 |
Gupta D , Lis CG . Pretreatment serum albumin as a predictor of cancer survival: A systematic review of the epidemiological literature[J]. Nutr J, 2010, 9 (1): 69- 85.
doi: 10.1186/1475-2891-9-69 |
| 31 |
Chandra RK . Nutrition and immunology: From the clinic to cellular biology and back again[J]. Proc Nutr Soc, 1999, 58 (3): 681- 683.
doi: 10.1017/S0029665199000890 |
| 32 | Alwarawrah Y , Kiernan K , MacIver NJ . Changes in nutritional status impact immune cell metabolism and function[J]. Front Immunol, 2018, 9 (1): 1055- 1069. |
| 33 |
Tang Y , Liu Z , Liang J , et al. Early post-operative serum albumin level predicts survival after curative nephrectomy for kidney cancer: A retrospective study[J]. BMC Urol, 2018, 18 (1): 111- 118.
doi: 10.1186/s12894-018-0427-3 |
| 34 |
Corcoran AT , Kaffenberger SD , Clark PE , et al. Hypoalbuminaemia is associated with mortality in patients undergoing cytoreductive nephrectomy[J]. BJU Int, 2015, 116 (3): 351- 357.
doi: 10.1111/bju.12897 |
| 35 |
Stone PC , Lund S . Predicting prognosis in patients with advanced cancer[J]. Ann Oncol, 2007, 18 (6): 971- 976.
doi: 10.1093/annonc/mdl343 |
| 36 |
Volpe A , Patard JJ . Prognostic factors in renal cell carcinoma[J]. World J Urol, 2010, 28 (3): 319- 327.
doi: 10.1007/s00345-010-0540-8 |
| [1] | 欧俊永,倪坤明,马潞林,王国良,颜野,杨斌,李庚午,宋昊东,陆敏,叶剑飞,张树栋. 肌层浸润性膀胱癌合并中高危前列腺癌患者的预后因素[J]. 北京大学学报(医学版), 2024, 56(4): 582-588. |
| [2] | 刘帅,刘磊,刘茁,张帆,马潞林,田晓军,侯小飞,王国良,赵磊,张树栋. 伴静脉癌栓的肾上腺皮质癌的临床治疗及预后[J]. 北京大学学报(医学版), 2024, 56(4): 624-630. |
| [3] | 杨捷,冯杰莉,张树栋,马潞林,郑清. 经食管超声心动图在肾切除术联合Mayo Ⅲ~Ⅳ级静脉瘤栓取栓术不同手术方式中的临床作用[J]. 北京大学学报(医学版), 2024, 56(4): 631-635. |
| [4] | 虞乐,邓绍晖,张帆,颜野,叶剑飞,张树栋. 具有低度恶性潜能的多房囊性肾肿瘤的临床病理特征及预后[J]. 北京大学学报(医学版), 2024, 56(4): 661-666. |
| [5] | 周泽臻,邓绍晖,颜野,张帆,郝一昌,葛力源,张洪宪,王国良,张树栋. 非转移性T3a肾细胞癌患者3年肿瘤特异性生存期预测[J]. 北京大学学报(医学版), 2024, 56(4): 673-679. |
| [6] | 方杨毅,李强,黄志高,陆敏,洪锴,张树栋. 睾丸鞘膜高分化乳头状间皮肿瘤1例[J]. 北京大学学报(医学版), 2024, 56(4): 741-744. |
| [7] | 曾媛媛,谢云,陈道南,王瑞兰. 脓毒症患者发生正常甲状腺性病态综合征的相关因素[J]. 北京大学学报(医学版), 2024, 56(3): 526-532. |
| [8] | 苏俊琪,王晓颖,孙志强. 舌鳞状细胞癌根治性切除术后患者预后预测列线图的构建与验证[J]. 北京大学学报(医学版), 2024, 56(1): 120-130. |
| [9] | 李建斌,吕梦娜,池强,彭一琳,刘鹏程,吴锐. 干燥综合征患者发生重症新型冠状病毒肺炎的早期预测[J]. 北京大学学报(医学版), 2023, 55(6): 1007-1012. |
| [10] | 刘欢锐,彭祥,李森林,苟欣. 基于HER-2相关基因构建风险模型用于膀胱癌生存预后评估[J]. 北京大学学报(医学版), 2023, 55(5): 793-801. |
| [11] | 薛子璇,唐世英,邱敏,刘承,田晓军,陆敏,董靖晗,马潞林,张树栋. 青年肾肿瘤伴瘤栓的临床病理特征及预后分析[J]. 北京大学学报(医学版), 2023, 55(5): 802-811. |
| [12] | 卢汉,张建运,杨榕,徐乐,李庆祥,郭玉兴,郭传瑸. 下颌牙龈鳞状细胞癌患者预后的影响因素[J]. 北京大学学报(医学版), 2023, 55(4): 702-707. |
| [13] | 时云飞,王豪杰,刘卫平,米岚,龙孟平,刘雁飞,赖玉梅,周立新,刁新婷,李向红. 血管免疫母细胞性T细胞淋巴瘤临床与分子病理学特征分析[J]. 北京大学学报(医学版), 2023, 55(3): 521-529. |
| [14] | 许云屹,苏征征,郑林茂,张孟尼,谭珺娅,杨亚蓝,张梦鑫,徐苗,陈铌,陈雪芹,周桥. 转录通读环状RNA rt-circ-HS促进低氧诱导因子1α表达和肾癌细胞增殖与侵袭[J]. 北京大学学报(医学版), 2023, 55(2): 217-227. |
| [15] | 朱晓娟,张虹,张爽,李东,李鑫,徐玲,李挺. 人表皮生长因子受体2低表达乳腺癌的临床病理学特征及预后[J]. 北京大学学报(医学版), 2023, 55(2): 243-253. |
|
||