北京大学学报(医学版) ›› 2023, Vol. 55 ›› Issue (2): 217-227. doi: 10.19723/j.issn.1671-167X.2023.02.004
转录通读环状RNA rt-circ-HS促进低氧诱导因子1α表达和肾癌细胞增殖与侵袭
许云屹1,苏征征1,郑林茂1,张孟尼1,谭珺娅1,2,杨亚蓝1,张梦鑫1,徐苗1,陈铌1,2,陈雪芹1,2,周桥1,2,*( )
)
- 1. 四川大学华西医院病理科,成都 610041
2. 四川大学华西医院病理研究室,成都 610041
Read-through circular RNA rt-circ-HS promotes hypoxia inducible factor 1α expression and renal carcinoma cell proliferation, migration and invasiveness
Yun-yi XU1,Zheng-zheng SU1,Lin-mao ZHENG1,Meng-ni ZHANG1,Jun-ya TAN1,2,Ya-lan YANG1,Meng-xin ZHANG1,Miao XU1,Ni CHEN1,2,Xue-qin CHEN1,2,Qiao ZHOU1,2,*( )
)
- 1. Department of Pathology, West China Hospital, Sichuan University, Chengdu 610041, China
2. Research Laboratory of Pathology, West China Hospital, Sichuan University, Chengdu 610041, China
摘要:
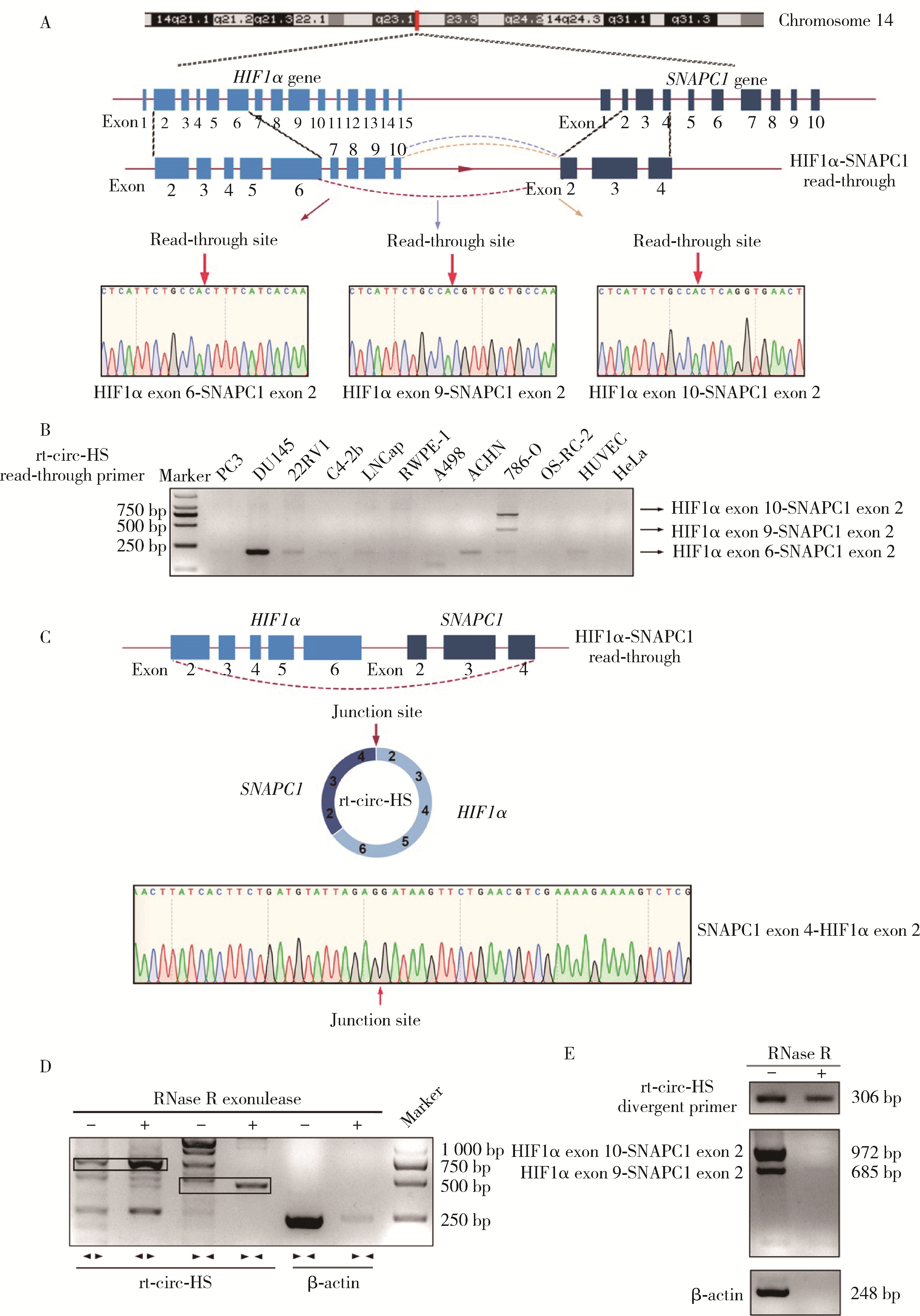
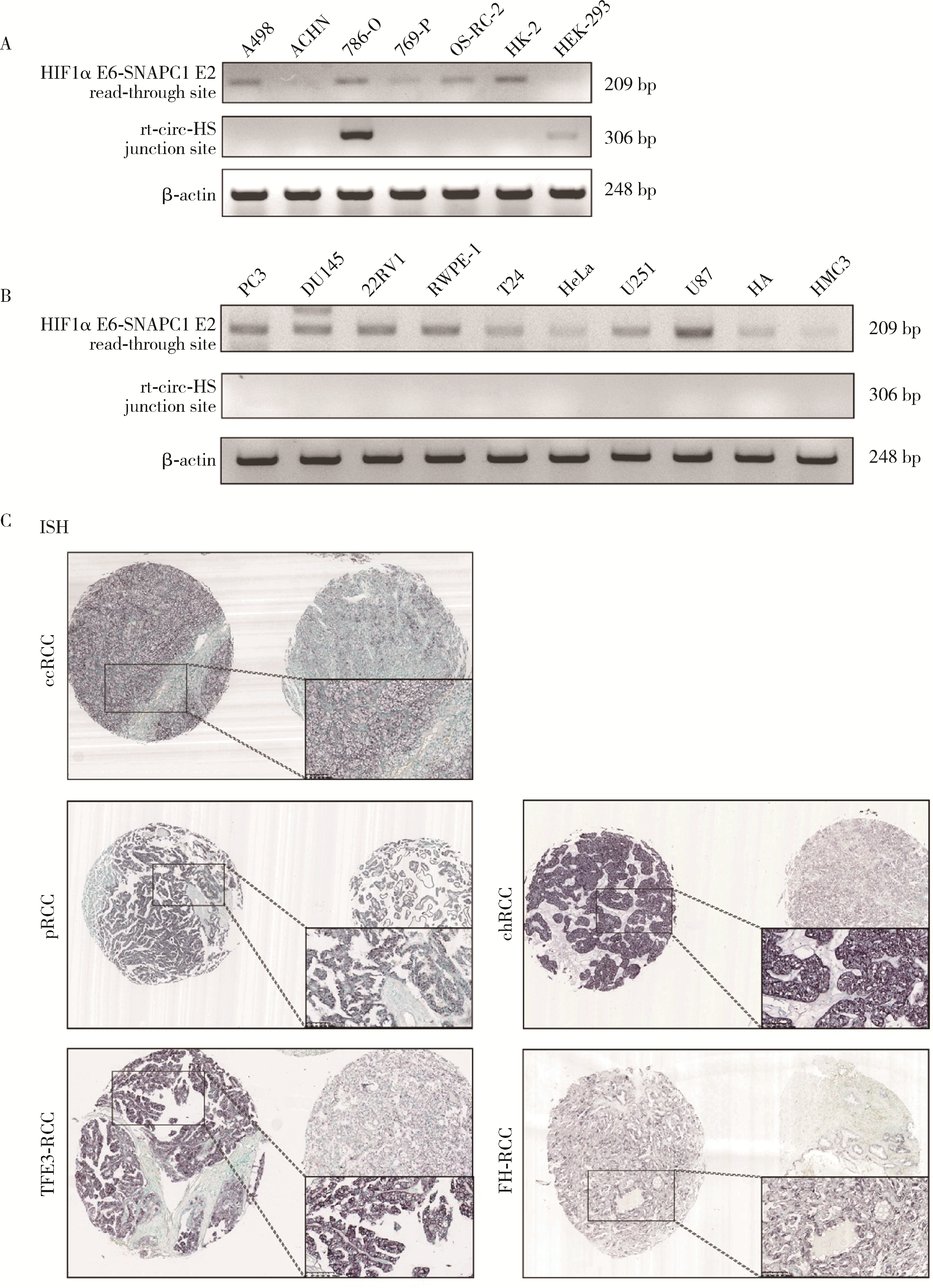
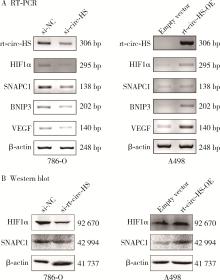
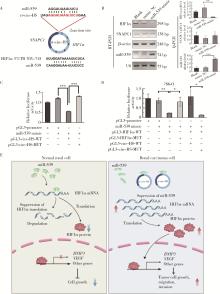
目的: 鉴定肾癌细胞中由染色体14q23上相邻基因低氧诱导因子1α(hypoxia inducible factor 1α,HIF1α)和小核RNA激活复合多肽(small nuclear RNA activating complex polypeptide 1, SNAPC1) 形成的转录通读RNA及转录通读环状RNA(read-through circular RNA HIF1α-SNAPC1, rt-circ-HS),研究rt-circ-HS在肾癌细胞及组织样本中的表达、对肾癌细胞生物学行为的影响以及对其亲本分子HIF1α的调控机制。方法: 逆转录PCR(reverse transcription-polymerase chain reaction, RT-PCR)和Sanger测序检测不同肿瘤细胞中由HIF1α-SNAPC1形成的转录通读RNA和rt-circ-HS的表达。构建不同类型的肾细胞癌(renal cell carcinoma,RCC)组织芯片共437例,用原位杂交检测rt-circ-HS表达。采用小干扰RNA(small interference RNA,si-RNA)和人工过表达质粒干预rt-circ-HS,用细胞计数实验(cell counting kit 8,CCK8)、EdU掺入实验、Transwell细胞迁移和细胞侵袭实验分别检测rt-circ-HS对肾癌细胞增殖、迁移和侵袭的影响。用RT-PCR和Western blot验证干预rt-circ-HS对亲本分子HIF1α和SNAPC1表达的影响。构建包含rt-circ-HS、HIF1α 3′端非翻译区(3′ untranslated region, 3′ UTR)与微小RNA 539(microRNA 539,miR-539)结合序列的野生型和突变型质粒,用双荧光素酶报告基因系统检测rt-circ-HS、HIF1α 3′ UTR与miR-539的结合。结果: 发现一个新的rt-circ-HS,由HIF1α外显子(exon) 6-SNAPC1 exon 2转录通读本产生,在肾癌细胞786-O中高表达。Sanger测序证实rt-circ-HS全长1 144 nt,包括HIF1α exon 2-exon 6和SNAPC1 exon 2-exon 4,是一个新的转录通读环状RNA。原位杂交结果显示,rt-circ-HS在RCC中阳性表达率为67.5%(295/437),在不同类型RCC中表达率不同。发现miR-539是HIF1α的转录后负调控分子;rt-circ-HS作为分子海绵与miR-539结合,竞争性抑制miR-539与HIF1α 3′ UTR的结合,解除其对HIF1α的转录后负调控作用,促进亲本分子HIF1α表达及肾癌细胞增殖、迁移和侵袭。结论: rt-circ-HS作为分子海绵结合miR-539,抑制其对HIF1α的负调控作用,促进亲本分子HIF1α表达及肾癌细胞增殖、迁移和侵袭。
中图分类号:
- R365
| 1 |
Zhou WY , Cai ZR , Liu J , et al. Circular RNA: Metabolism, functions and interactions with proteins[J]. Mol Cancer, 2020, 19 (1): 172.
doi: 10.1186/s12943-020-01286-3 |
| 2 |
Chen Q , Liu T , Bao Y , et al. CircRNA cRAPGEF5 inhibits the growth and metastasis of renal cell carcinoma via the miR-27a-3p/TXNIP pathway[J]. Cancer Lett, 2020, 469, 68- 77.
doi: 10.1016/j.canlet.2019.10.017 |
| 3 |
Mao W , Wang K , Xu B , et al. ciRS-7 is a prognostic biomarker and potential gene therapy target for renal cell carcinoma[J]. Mol Cancer, 2021, 20 (1): 142- 155.
doi: 10.1186/s12943-021-01443-2 |
| 4 |
van Zonneveld AJ , Kolling M , Bijkerk R , et al. Circular RNAs in kidney disease and cancer[J]. Nat Rev Nephrol, 2021, 17 (12): 814- 826.
doi: 10.1038/s41581-021-00465-9 |
| 5 |
Zhang Y , Gong M , Yuan H , et al. Chimeric transcript generated by cis-splicing of adjacent genes regulates prostate cancer cell proliferation[J]. Cancer Discov, 2012, 2 (7): 598- 607.
doi: 10.1158/2159-8290.CD-12-0042 |
| 6 |
Grosso AR , Leite AP , Carvalho S , et al. Pervasive transcription read-through promotes aberrant expression of oncogenes and RNA chimeras in renal carcinoma[J]. Elife, 2015, 4, e09214.
doi: 10.7554/eLife.09214 |
| 7 |
Pflueger D , Mittmann C , Dehler S , et al. Functional characterization of BC039389-GATM and KLK4-KRSP1 chimeric read-through transcripts which are up-regulated in renal cell cancer[J]. BMC Genomics, 2015, 16 (1): 247.
doi: 10.1186/s12864-015-1446-z |
| 8 |
Chen N , Zhou Q . Constructing tissue microarrays without prefabricating recipient blocks: A novel approach[J]. Am J Clin Pathol, 2005, 124 (1): 103- 107.
doi: 10.1309/LHCJRFBUH8Q6QD3N |
| 9 |
Turajlic S , Swanton C , Boshoff C . Kidney cancer: The next decade[J]. J Exp Med, 2018, 215 (10): 2477- 2479.
doi: 10.1084/jem.20181617 |
| 10 |
Siegel RL , Miller KD , Fuchs HE , et al. Cancer statistics 2022[J]. CA Cancer J Clin, 2022, 72 (1): 7- 33.
doi: 10.3322/caac.21708 |
| 11 |
Garje R , Elhag D , Yasin HA , et al. Comprehensive review of chromophobe renal cell carcinoma[J]. Crit Rev Oncol Hematol, 2021, 160, 103287.
doi: 10.1016/j.critrevonc.2021.103287 |
| 12 |
Ji SQ , Su XL , Cheng WL , et al. Down-regulation of CD74 inhi-bits growth and invasion in clear cell renal cell carcinoma through HIF-1α pathway[J]. Urol Oncol, 2014, 32 (2): 153- 161.
doi: 10.1016/j.urolonc.2012.09.013 |
| 13 |
Hu CJ , Wang LY , Chodosh LA , et al. Differential roles of hypo-xia-inducible factor 1alpha (HIF-1alpha) and HIF-2alpha in hypoxic gene regulation[J]. Mol Cell Biol, 2003, 23 (24): 9361- 9374.
doi: 10.1128/MCB.23.24.9361-9374.2003 |
| 14 |
Shen C , Beroukhim R , Schumacher SE , et al. Genetic and functional studies implicate HIF1alpha as a 14q kidney cancer suppressor gene[J]. Cancer Discov, 2011, 1 (3): 222- 235.
doi: 10.1158/2159-8290.CD-11-0098 |
| 15 | Shinojima T , Oya M , Takayanagi A , et al. Renal cancer cells lacking hypoxia inducible factor (HIF)-1alpha expression maintain vascular endothelial growth factor expression through HIF-2alpha[J]. Carcinogenesis, 2007, 28 (3): 529- 536. |
| 16 |
Swiatek M , Jancewicz I , Kluebsoongnoen J , et al. Various forms of HIF-1alpha protein characterize the clear cell renal cell carcinoma cell lines[J]. IUBMB Life, 2020, 72 (6): 1220- 1232.
doi: 10.1002/iub.2281 |
| 17 |
Vidal AF . Read-through circular RNAs reveal the plasticity of RNA processing mechanisms in human cells[J]. RNA Biol, 2020, 17 (12): 1823- 1826.
doi: 10.1080/15476286.2020.1805233 |
| 18 |
Yang X , Ye T , Liu H , et al. Expression profiles, biological functions and clinical significance of circRNAs in bladder cancer[J]. Mol Cancer, 2021, 20 (1): 4.
doi: 10.1186/s12943-020-01300-8 |
| 19 |
Wu X , Zhou J , Zhao L , et al. CircCYP24A1 hampered malignant phenotype of renal cancer carcinoma through modulating CMTM-4 expression via sponging miR-421[J]. Cell Death Dis, 2022, 13 (2): 190.
doi: 10.1038/s41419-022-04623-0 |
| 20 |
Wang X , Xing L , Yang R , et al. The circACTN4 interacts with FUBP1 to promote tumorigenesis and progression of breast cancer by regulating the expression of proto-oncogene MYC[J]. Mol Cancer, 2021, 20 (1): 91.
doi: 10.1186/s12943-021-01383-x |
| 21 |
Abdelmohsen K , Panda AC , Munk R , et al. Identification of HuR target circular RNAs uncovers suppression of PABPN1 translation by CircPABPN1[J]. RNA Biol, 2017, 14 (3): 361- 369.
doi: 10.1080/15476286.2017.1279788 |
| 22 |
Khan FA , Nsengimana B , Khan NH , et al. Chimeric peptides/proteins encoded by circRNA: An update on mechanisms and functions in human cancers[J]. Front Oncol, 2022, 12, 781270.
doi: 10.3389/fonc.2022.781270 |
| 23 |
Pintarelli G , Dassano A , Cotroneo CE , et al. Read-through transcripts in normal human lung parenchyma are down-regulated in lung adenocarcinoma[J]. Oncotarget, 2016, 7 (19): 27889- 27898.
doi: 10.18632/oncotarget.8556 |
| 24 |
Choi ES , Lee H , Lee CH , et al. Overexpression of KLHL23 protein from read-through transcription of PHOSPHO2-KLHL23 in gastric cancer increases cell proliferation[J]. FEBS Open Bio, 2016, 6 (11): 1155- 1164.
doi: 10.1002/2211-5463.12136 |
| 25 |
Wang L , Xiong X , Yao Z , et al. Chimeric RNA ASTN2-PAPPA(as) aggravates tumor progression and metastasis in human esophageal cancer[J]. Cancer Lett, 2021, 501, 1- 11.
doi: 10.1016/j.canlet.2020.10.052 |
| 26 |
Qin F , Zhang Y , Liu J , et al. SLC45A3-ELK4 functions as a long non-coding chimeric RNA[J]. Cancer Lett, 2017, 404, 53- 61.
doi: 10.1016/j.canlet.2017.07.007 |
| [1] | 周泽臻,邓绍晖,颜野,张帆,郝一昌,葛力源,张洪宪,王国良,张树栋. 非转移性T3a肾细胞癌患者3年肿瘤特异性生存期预测[J]. 北京大学学报(医学版), 2024, 56(4): 673-679. |
| [2] | 沈棋,刘亿骁,何群. 肾黏液样小管状和梭形细胞癌的临床病理特点及预后[J]. 北京大学学报(医学版), 2023, 55(2): 276-282. |
| [3] | 张铨,宋海峰,马冰磊,张喆楠,周朝晖,李傲林,刘军,梁磊,朱时雨,张骞. 术前预后营养指数可作为预测非转移性肾细胞癌预后的指标[J]. 北京大学学报(医学版), 2023, 55(1): 149-155. |
| [4] | 博尔术,洪鹏,张宇,邓绍晖,葛力源,陆敏,李楠,马潞林,张树栋. 乳头状肾细胞癌的临床病理特征和预后分析[J]. 北京大学学报(医学版), 2022, 54(4): 615-620. |
| [5] | 周鑫,李文智. 肾细胞癌极致保肾时代的冷思考[J]. 北京大学学报(医学版), 2022, 54(4): 595-598. |
| [6] | 田雨,程晓悦,贺慧颖,王国良,马潞林. 肾细胞癌合并尿路瘤栓的临床病理特征: 6例报道及文献回顾[J]. 北京大学学报(医学版), 2021, 53(5): 928-932. |
| [7] | 韩松辰,黄子雄,刘慧鑫,徐涛. 单侧肾细胞癌根治性切除术后的肾功能代偿[J]. 北京大学学报(医学版), 2021, 53(4): 680-685. |
| [8] | 赵勋,颜野,黄晓娟,董靖晗,刘茁,张洪宪,刘承,马潞林. 癌栓粘连血管壁对非转移性肾细胞癌合并下腔静脉癌栓患者手术及预后的影响[J]. 北京大学学报(医学版), 2021, 53(4): 665-670. |
| [9] | 孙争辉,黄晓娟,董靖晗,刘茁,颜野,刘承,马潞林. 临床T1期肾细胞癌肾窦侵犯的危险因素[J]. 北京大学学报(医学版), 2021, 53(4): 659-664. |
| [10] | 于妍斐,何世明,吴宇财,熊盛炜,沈棋,李妍妍,杨风,何群,李学松. 延胡索酸水合酶缺陷型肾细胞癌的临床病理特征及预后[J]. 北京大学学报(医学版), 2021, 53(4): 640-646. |
| [11] | 张晓鹏,黄子雄,于路平,张晓威,李清,刘士军,徐涛. 小肾细胞癌的临床与病理特征分析[J]. 北京大学学报(医学版), 2019, 51(4): 623-627. |
| [12] | 黄子雄,杜依青,张晓鹏,刘士军,徐涛. 肾细胞癌骨转移的临床与病理分析[J]. 北京大学学报(医学版), 2018, 50(5): 811-815. |
| [13] | 丁振山,邱敏,徐梓程,肖若陶,葛力源,马潞林. 乳头状肾细胞癌合并癌栓患者的临床病理分析[J]. 北京大学学报(医学版), 2018, 50(5): 805-810. |
| [14] | 孙静,宋卫东,闫思源,席志军. 氯喹抑制肾癌细胞活性促进舒尼替尼诱导的细胞凋亡[J]. 北京大学学报(医学版), 2018, 50(5): 778-784. |
|
||