北京大学学报(医学版) ›› 2021, Vol. 53 ›› Issue (2): 371-377. doi: 10.19723/j.issn.1671-167X.2021.02.023
生物活性玻璃对人脐静脉血管内皮细胞增殖及成血管的作用
- 北京大学口腔医学院·口腔医院,牙体牙髓科 国家口腔疾病临床医学研究中心 口腔数字化医疗技术和材料国家工程实验室 口腔数字医学北京市重点实验室, 北京 100081
Effects of bioactive glass on proliferation, differentiation and angiogenesis of human umbilical vein endothelial cells
HUANG Li-dong,GONG Wei-yu,DONG Yan-mei( )
)
- Department of Cariology and Endodontology, Peking University School and Hospital of Stomatology & National Clinical Research Center for Oral Diseases & National Engineering Laboratory for Digital and Material Technology of Stomatology & Beijing Key Laboratory of Digital Stomatology, Beijing 100081, China
摘要:
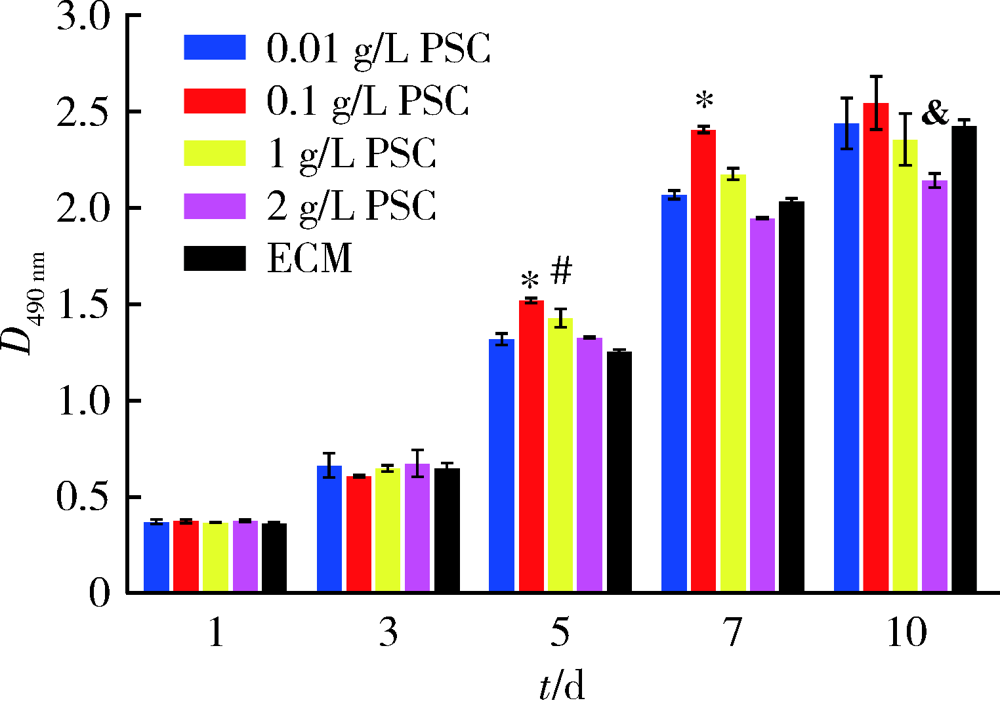
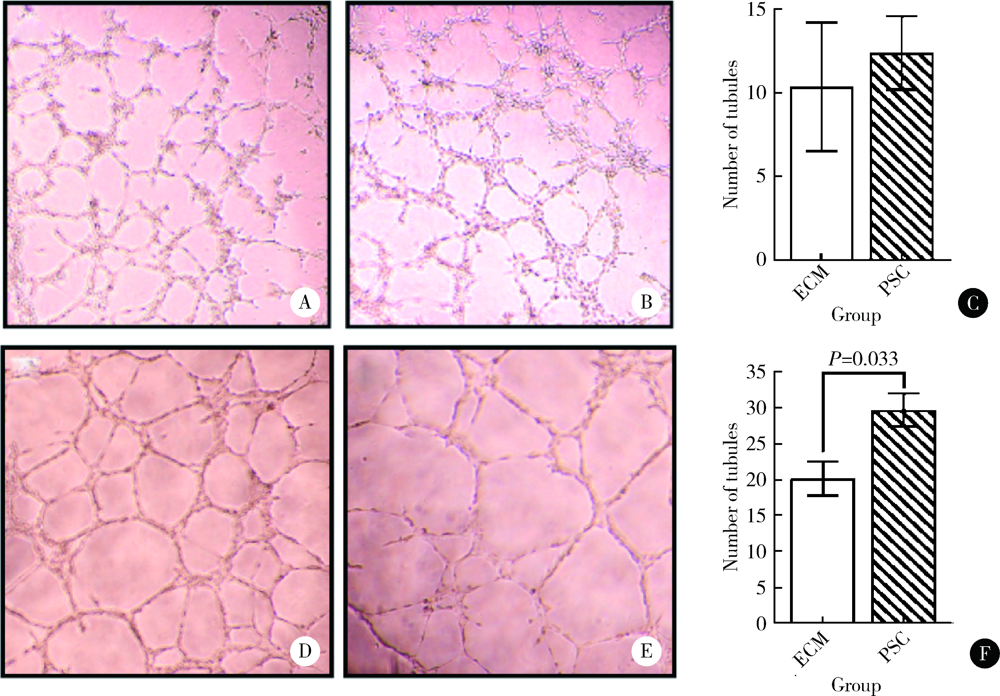
目的: 研究以植酸为前驱体制备的生物活性玻璃(phytic acid derived bioactive P2O5-SiO2-CaO gel-glasses, PSC)对人脐静脉内皮细胞(human umbilical vein endothelial cells, HUVECs)增殖、分化及体外成血管的作用。方法: 用内皮细胞培养基(endothelial cell medium, ECM)按梯度浓度(0.01、0.1、1和2 g/L)分别制备PSC浸提液培养HUVECs,在第1、3、5、7、10天采用甲基噻唑基四唑[(4,5-dimethylthiazol-2-yl)2,5-diphenyltetrazolium bromide, MTT]法对细胞的增殖能力进行检测,并与ECM培养的对照组HUVECs进行比较。根据增殖实验结果,选择最适宜HUVECs生长的PSC浸提液浓度用于后续实验。后续实验分为两组:实验组用PSC浸提液培养HUVECs(PSC组),对照组用ECM培养HUVECs(ECM组)。采用实时反转录聚合酶链反应(real-time reverse transcription-polymerase chain reaction, real-time RT-PCR)于第2、4、7天检测血管内皮生长因子(vascular endothelial growth factor, VEGF)、碱性成纤维细胞生长因子(basic fibroblast growth factor, bFGF)的基因表达;通过小管形成实验观察第 4、10小时PSC浸提液对HUVECs形成小管的形态与数目的影响,并用Image J软件定量分析。结果: 用MTT法筛选最适生长浓度,结果提示0.1 g/L PSC浸提液对HUVECs的增殖促进作用最为显著,在第5、7天时细胞光密度值显著高于其他浓度实验组及对照组(P<0.05)。0.1 g/L PSC组可显著上调HUVECs成血管基因VEGF、bFGF的表达(P<0.05);第4天时,PSC组VEGF、bFGF的基因表达量分别是ECM组的1.59和1.45倍;第7天时,PSC组VEGF、bFGF的基因表达量分别是ECM组的1.98和1.37倍。在小管形成实验第10小时,PSC组小管的成熟度与密度优于ECM组,PSC组的小管数目(29.63±2.29)高于ECM组(20.13±2.36),差异有统计学意义(P<0.05)。结论: PSC具有促进HUVECs增殖、分化及体外成血管的作用。
中图分类号:
- R782.12
| [1] |
Hoppe A, Güldal NS, Boccaccini AR. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics[J]. Biomaterials, 2011,32(11):2757-2774.
pmid: 21292319 |
| [2] |
Keothongkham K, Charoenphandhu N, Thongbunchoo J, et al. Evaluation of bioactive glass incorporated poly(caprolactone)-poly(vinyl alcohol) matrix and the effect of BMP-2 modification[J]. Mater Sci Eng C Mater Biol Appl, 2017,74:47-54.
pmid: 28254319 |
| [3] |
Nommeots-Nomm A, Labbaf S, Devlin A, et al. Highly degradable porous melt-derived bioactive glass foam scaffolds for bone regeneration[J]. Acta Biomater, 2017,57:449-461.
pmid: 28457960 |
| [4] |
Fujishiro Y, Hench LL, Oonishi H. Quantitative rates of in vivo bone generation for Bioglass and hydroxyapatite particles as bone graft substitute[J]. J Mater Sci Mater Med, 1997,8(11):649-652.
pmid: 15348815 |
| [5] |
Santos MI, Reis RL. Vascularization in bone tissue engineering: physiology, current strategies, major hurdles and future challenges[J]. Macromol Biosci, 2010,10(1):12-27.
pmid: 19688722 |
| [6] |
Rust KR, Singleton GT, Wilson J, et al. Bioglass middle ear prosjournal: long-term results[J]. Am J Otol, 1996,17(3):371-374.
pmid: 8817012 |
| [7] | Hench LL, Splinter RJ, Allen WC, et al. Bonding mechanisms at the interface of ceramic prosthetic materials[J]. J Biomed Mater Res A, 1971,5(6):117-141. |
| [8] |
Stanley HR, Hall MB, Clark AE, et al. Using 45S5 bioglass cones as endosseous ridge maintenance implants to prevent alveolar ridge resorption: a 5-year evaluation[J]. Int J Oral Maxillofac Implants, 1997,12(1):95-105.
pmid: 9048461 |
| [9] |
Keles GC, Cetinkaya BO, Albayrak D, et al. Comparison of platelet pellet and bioactive glass in periodontal regenerative therapy[J]. Acta Odontol Scand, 2009,64(6):327-333.
pmid: 17123908 |
| [10] |
Rahaman MN, Day DE, Bal BS, et al. Bioactive glass in tissue engineering[J]. Acta Biomater, 2011,7(6):2355-2373.
doi: 10.1016/j.actbio.2011.03.016 pmid: 21421084 |
| [11] |
Day RM, Boccaccini AR, Shurey S, et al. Assessment of polyglycolic acid mesh and bioactive glass for soft-tissue engineering scaffolds[J]. Biomaterials, 2004,25(27):5857-5866.
pmid: 15172498 |
| [12] | Day RM. Bioactive glass stimulates the secretion of angiogenic growth factors and angiogenesis in vitro[J]. Tissue Eng, 2005,11(5/6):768. |
| [13] |
Keshaw H, Forbes A, Day RM. Release of angiogenic growth factors from cells encapsulated in alginate beads with bioactive glass[J]. Biomaterials, 2005,26(19):4171-4179.
pmid: 15664644 |
| [14] |
Andrade AL, Andrade SP, Domingues RZ. In vivo performance of a sol-gel glass-coated collagen[J]. J Biomed Mater Res B Appl Biomater, 2010,79(1):122-128.
pmid: 16615070 |
| [15] |
Ghosh SK, Nandi SK, Kundu B, et al. In vivo response of porous hydroxyapatite and beta-tricalcium phosphate prepared by aqueous solution combustion method and comparison with bioglass scaffolds[J]. J Biomed Mater Res B Appl Biomater, 2008,86(1):217-227.
pmid: 18161811 |
| [16] |
Nandi SK, Kundu B, Datta S, et al. The repair of segmental bone defects with porous bioglass: an experimental study in goat[J]. Res Vet Sci, 2009,86(1):162-173.
pmid: 18602125 |
| [17] |
Ross EA, Batich CD, Clapp WL, et al. Tissue adhesion to bioactive glass-coated silicone tubing in a rat model of peritoneal dialysis catheters and catheter tunnels[J]. Kidney Int, 2003,63(2):702-708.
pmid: 12631137 |
| [18] |
Choi HY, Lee JE, Park HJ, et al. Effect of synthetic bone glass particulate on the fibrovascularization of porous polyethylene orbital implants[J]. Ophthalmic Plast Reconstr Surg, 2006,22(2):121-125.
doi: 10.1097/01.iop.0000197022.19166.dd pmid: 16550057 |
| [19] |
Day RM, Maquet V, Boccaccini AR, et al. In vitro and in vivo analysis of macroporous biodegradable poly (d,l-lactide-co-glyco-lide) scaffolds containing bioactive glass[J]. J Biomed Mater Res A, 2005,75(4):778-787.
pmid: 16082717 |
| [20] |
Keshaw H, Georgiou G, Blaker JJ, et al. Assessment of polymer/bioactive glass-composite microporous spheres for tissue regeneration applications[J]. Tissue Eng Part A, 2009,15(7):1451-1461.
pmid: 19061428 |
| [21] |
Lei B, Shin KH, Noh DY, et al. Sol-gel derived nanoscale bioactive glass (NBG) particles reinforced poly (ε-caprolactone) composites for bone tissue engineering[J]. Mater Sci Eng C Mater Biol Appl, 2013,33(3):1102-1108.
pmid: 23827548 |
| [22] |
Li A, Qiu D. Phytic acid derived bioactive CaO-P2O5-SiO2 gel-glasses[J]. J Mater Sci Mater Med, 2011,22(12):2685-2691.
pmid: 22042461 |
| [23] |
Zhu T, Ren H, Li A, et al. Novel bioactive glass based injectable bone cement with improved osteoinductivity and its in vivo evaluation[J]. Sci Rep, 2017,7(1):3622.
pmid: 28620229 |
| [24] |
Rouwkema J, Khademhosseini A. Vascularization and angiogenesis in tissue engineering: beyond creating static networks[J]. Trends Biotechnol, 2016,34(9):733-745.
doi: 10.1016/j.tibtech.2016.03.002 pmid: 27032730 |
| [25] |
Mao C, Chen X, Miao G, et al. Angiogenesis stimulated by novel nanoscale bioactive glasses[J]. Biomed Mater, 2015,10(2):025005.
pmid: 25805509 |
| [26] | Cui CY, Wang SN, Ren HH, et al. Regeneration of dental-pulp complex-like tissue using phytic acid derived bioactive glasses[J]. RSC Adv, 2017,7(36):22063-22070. |
| [27] |
Gorustovich AA, Roether JA, Boccaccini AR. Roether. Effect of bioactive glasses on angiogenesis: a review of in vitro and in vivo evidences[J]. Tissue Eng Part B Rev, 2010,16(2):199-207.
pmid: 19831556 |
| [28] |
Leu A, Leach JK. Proangiogenic potential of a collagen/bioactive glass substrate[J]. Pharm Res, 2008,25(5):1222-1229.
pmid: 18049878 |
| [29] |
Jones JR, Sepulveda P, Hench LL. Dose-dependent behavior of bioactive glass dissolution[J]. J Biomed Mater Res, 2010,58(6):720-726.
pmid: 11745526 |
| [30] | O’Donnell MD, Watts SJ, Law RV, et al. Effect of P2O5 content in two series of soda lime phosphosilicate glasses on structure and properties. Part Ⅰ: NMR[J]. J Non Cryst Solids, 2008,354(30):3554-3560. |
| [31] |
Shie MY, Ding SJ, Chang HC. The role of silicon in osteoblast-like cell proliferation and apoptosis[J]. Acta Biomater, 2011,7(6):2604-2614.
doi: 10.1016/j.actbio.2011.02.023 pmid: 21345382 |
| [32] |
Maeno S, Niki Y, Matsumoto H, et al. The effect of calcium ion concentration on osteoblast viability, proliferation and differentiation in monolayer and 3D culture[J]. Biomaterials, 2005,26(23):4847-4855.
pmid: 15763264 |
| [33] |
Zhai W, Lu H, Chen L, et al. Silicate bioceramics induce angiogenesis during bone regeneration[J]. Acta Biomater, 2012,8(1):341-349.
pmid: 21964215 |
| [34] |
Li H, Chang J. Stimulation of proangiogenesis by calcium silicate bioactive ceramic[J]. Acta Biomater, 2013,9(2):5379-5389.
doi: 10.1016/j.actbio.2012.10.019 pmid: 23088882 |
| [35] |
Schwarz K. A bound form of silicon in glycosaminoglycans and polyuronides[J]. Proc Natl Acad Sci USA, 1973,70(5):1608-1612.
pmid: 4268099 |
| [1] | 陈晓颖,张一,李雨柯,唐琳,刘玉华. 不同种类聚合物对猪小肠黏膜下层支架仿生矿化的影响[J]. 北京大学学报(医学版), 2024, 56(1): 17-24. |
| [2] | 李雨柯,王梅,唐琳,刘玉华,陈晓颖. 不同pH值对脱细胞小肠黏膜下层海绵支架螯合锶离子的影响[J]. 北京大学学报(医学版), 2023, 55(1): 44-51. |
| [3] | 郭若兰,黄桂彬,龙赟子,董艳梅. 新型生物活性玻璃促进人工牙本质龋再矿化的作用[J]. 北京大学学报(医学版), 2023, 55(1): 82-87. |
| [4] | 敖英芳,曹宸喜. 解析与重塑软骨组织修复再生微环境[J]. 北京大学学报(医学版), 2021, 53(5): 819-822. |
| [5] | 李秋菊,宫玮玉,董艳梅. 生物活性玻璃预处理对牙本质粘接界面耐久性的影响[J]. 北京大学学报(医学版), 2020, 52(5): 931-937. |
| [6] | 王梅, 李博文, 王思雯, 刘玉华. 猪小肠黏膜下层海绵的制备及促成骨作用[J]. 北京大学学报(医学版), 2020, 52(5): 952-958. |
| [7] | 梁晨,张维宇,胡浩,王起,方志伟,许克新. 膀胱扩大术两种不同术式的疗效及并发症比较[J]. 北京大学学报(医学版), 2019, 51(2): 293-297. |
| [8] | 李榕,陈科龙,王勇,刘云松,周永胜,孙玉春. 骨组织工程支架3D打印系统的建立与支架宏微结构精度的可控性评价[J]. 北京大学学报(医学版), 2019, 51(1): 115-119. |
| [9] | 龙赟子,刘思毅,李稳,董艳梅. 生物活性玻璃盖髓剂的理化性质[J]. 北京大学学报(医学版), 2018, 50(5): 887-891. |
| [10] | 王子成,程立,吕同德,苏黎,林健,周利群. 炎症因子预处理的脂肪干细胞可明显抑制外周血单个核细胞增殖[J]. 北京大学学报(医学版), 2018, 50(4): 590-594. |
| [11] | 朱林,王聿栋,董艳梅,陈晓峰. 缓释米诺环素的介孔纳米生物玻璃载药系统[J]. 北京大学学报(医学版), 2018, 50(2): 249-255. |
| [12] | 宫玮玉,刘绍清,董艳梅,高学军,陈晓峰. 纳米生物活性玻璃促进兔颅骨临界骨缺损修复[J]. 北京大学学报(医学版), 2018, 50(1): 42-48. |
| [13] | 刘颖君,欧阳翔英,王宇光,吕培军,安娜. 生长停滞特异性蛋白6在牙龈卟啉单胞菌脂多糖诱导内皮细胞黏附因子及趋化因子表达中的作用[J]. 北京大学学报(医学版), 2018, 50(1): 20-25. |
| [14] | 李皓,刘玉华,罗志强. 生物活性玻璃用于缓解活髓牙全冠预备后敏感的效果评价[J]. 北京大学学报(医学版), 2017, 49(4): 709-713. |
| [15] | 隋华欣, 吕培军, 王宇光, 王勇, 孙玉春. 低能量激光照射对人脂肪基质细胞增殖分化的影响[J]. 北京大学学报(医学版), 2017, 49(2): 337-343. |
|
||