北京大学学报(医学版) ›› 2021, Vol. 53 ›› Issue (2): 364-370. doi: 10.19723/j.issn.1671-167X.2021.02.022
两种可吸收生物膜联合去蛋白牛骨基质植入犬拔牙窝成骨的影像学评价
- 北京大学口腔医学院·口腔医院,修复科 国家口腔疾病临床医学研究中心 口腔数字化医疗技术和材料国家工程实验室 口腔数字医学北京市重点实验室,北京 100081
Efficacy of two barrier membranes and deproteinized bovine bone mineral on bone regeneration in extraction sockets: A microcomputed tomographic study in dogs
WANG Si-wen,YOU Peng-yue,LIU Yu-hua( ),WANG Xin-zhi,TANG Lin,WANG Mei
),WANG Xin-zhi,TANG Lin,WANG Mei
- Department of Prosthodontics, Peking University School and Hospital of Stomatology & National Clinical Research Center for Oral Diseases & National Engineering Laboratory for Digital and Material Technology of Stomatology & Beijing Key Laboratory of Digital Stomatology, Beijing 100081, China
摘要:
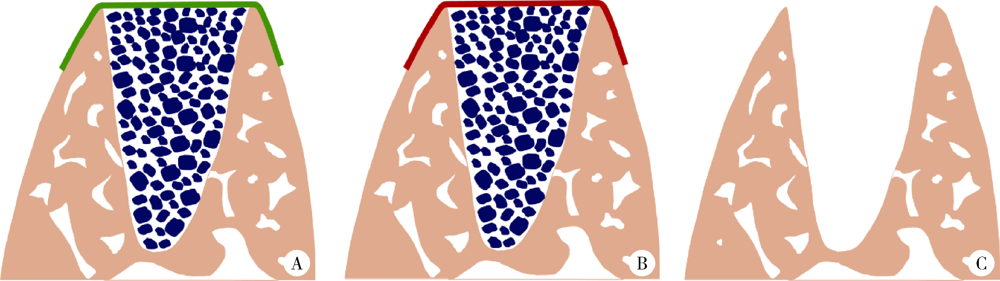
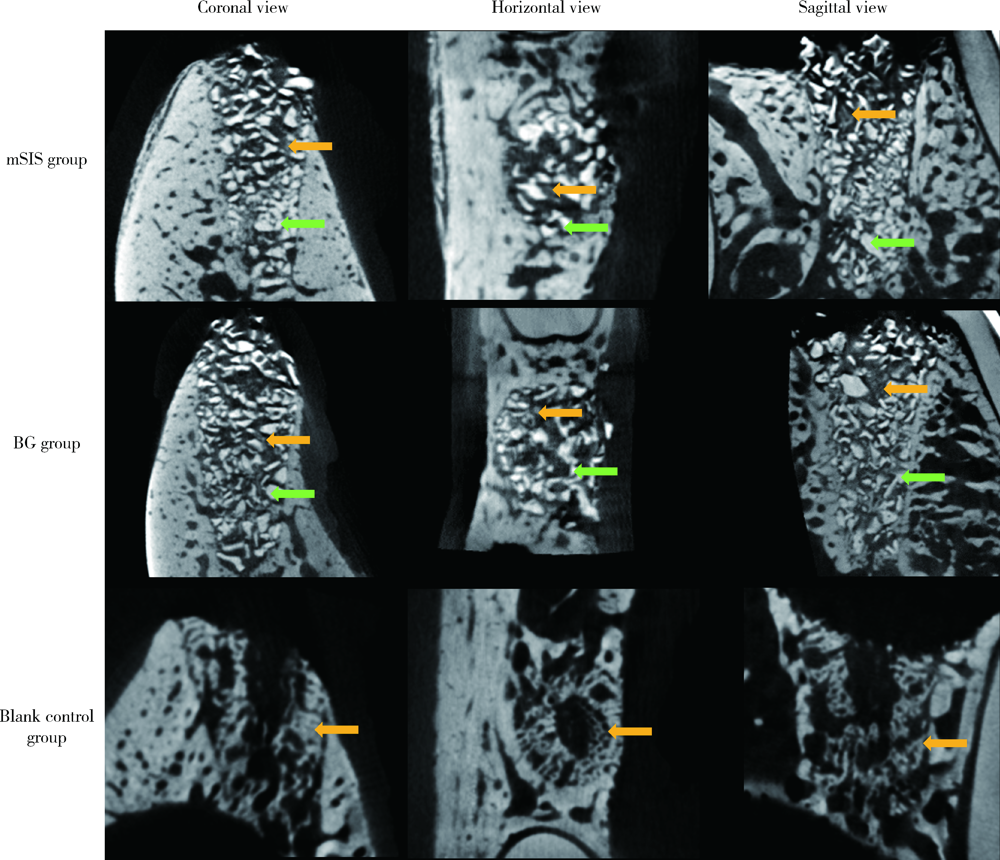
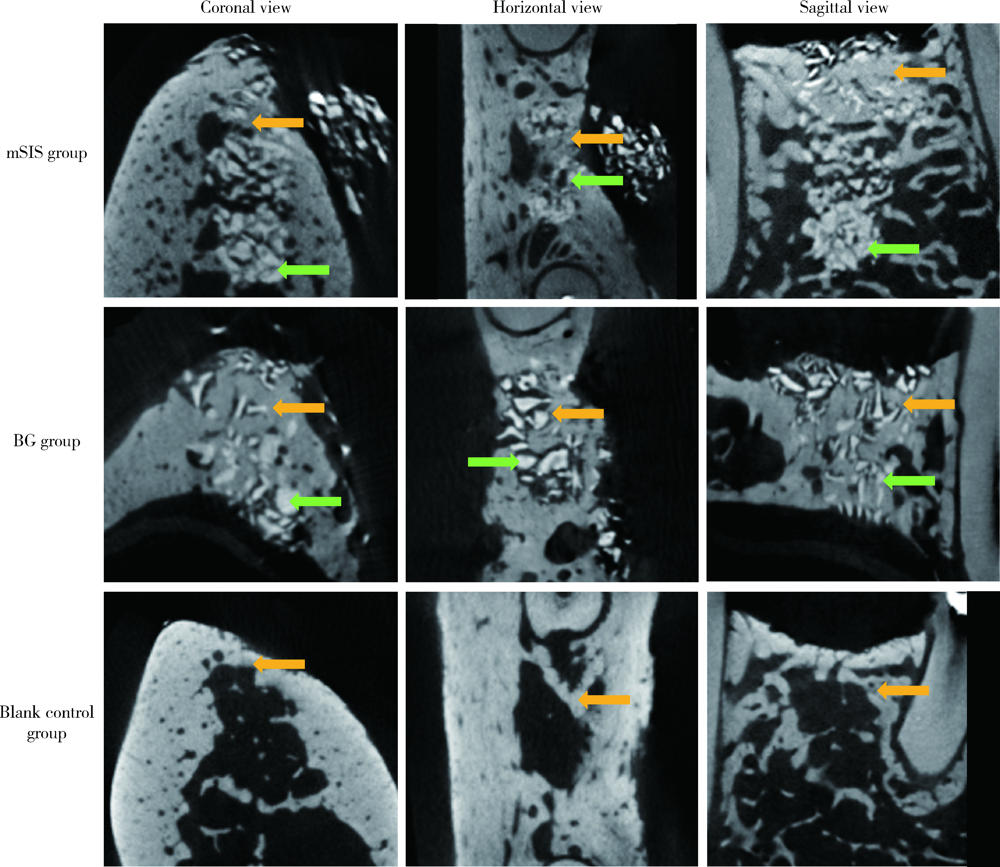
目的: 建立犬拔牙窝模型,采用影像学分析方法评价拔牙窝内植入去蛋白牛骨基质骨粉颗粒Bio-Oss®(简称Bio-Oss骨粉)并覆盖复层猪小肠黏膜下层膜(multilaminated small intestinal submucosa membrane, mSIS)或可吸收胶原膜Bio-Gide® (简称Bio-Gide膜), 愈合4周和12周后的牙槽窝内成骨效果。方法: 拔除3只比格犬双侧上下颌共计18颗前磨牙的远中根,得到18个拔牙窝,随机平均分为3大组,并分别对各拔牙窝组进行以下操作:(1)植入Bio-Oss骨粉并覆盖mSIS膜(mSIS组),(2)植入Bio-Oss骨粉并覆盖Bio-Gide膜(BG组),(3)自然愈合(空白对照组)。每大组各随机平均分为2个小组,分别于手术后4周和12周取样进行微计算机体层扫描(micro-computed tomograph, Micro-CT), 检测评价各组牙槽窝内新骨的生长情况,比较mSIS膜和Bio-Gide膜对拔牙窝内骨再生的影响。结果: Micro-CT分析显示,mSIS组和BG组在术后4周和12周的新生骨容积比均显著高于空白对照组(P<0.05),其中mSIS组略高于BG组,但两组间差异无统计学意义(P>0.05)。术后4周mSIS组和BG组的牙槽窝冠1/3区域新生骨容积比例显著高于中1/3及根1/3区域(P<0.05)。术后4周各组的新生骨密度值相近(P>0.05),术后12周时mSIS组和BG组的新生骨密度值均显著高于对照组(P<0.05)。术后4周和12周mSIS组和BG组的新生骨小梁的数量以及排列紧凑程度明显优于空白对照组(P<0.05),而mSIS略优于BG组,但两组间差异无统计学意义(P>0.05)。各组间骨小梁厚度的差异无统计学意义(P>0.05)。结论: 两种屏障膜联合去蛋白牛骨基质植入拔牙窝内有利于新骨再生,mSIS膜与Bio-Gide膜的应用效果相似。
中图分类号:
- R782.1
| [1] |
Ersanli S, Olgac V, Leblebicioglu B. Histologic analysis of alveolar bone following guided bone regeneration[J]. J Periodontol, 2004,75(5):750-756.
doi: 10.1902/jop.2004.75.5.750 pmid: 15212358 |
| [2] | Chiapasco M, Zaniboni M. Clinical outcomes of GBR procedures to correct peri-implant dehiscences and fenestrations: a systematic review[J]. Clin Oral Implants Res, 2009,20(Suppl 4):113-123. |
| [3] |
Oikarinen KS, Sandor GK, Kainulainen VT, et al. Augmentation of the narrow traumatized anterior alveolar ridge to facilitate dental implant placement[J]. Dent Traumatol, 2003,19(1):19-29.
pmid: 12656851 |
| [4] |
Amler MH. The time sequence of tissue regeneration in human extraction wounds[J]. Oral Surg Oral Med Oral Pathol, 1969,27(3):309-318.
pmid: 5251474 |
| [5] |
Nyman S, Lang NP, Buser D, et al. Bone regeneration adjacent to titanium dental implants using guided tissue regeneration: a report of two cases[J]. Int J Oral Maxillofac Implants, 1990,5(1):9-14.
pmid: 2391139 |
| [6] |
MacBeth N, Trullenque-Eriksson A, Donos N, et al. Hard and soft tissue changes following alveolar ridge preservation: a syste-matic review[J]. Clin Oral Implants Res, 2017,28(8):982-1004.
doi: 10.1111/clr.12911 pmid: 27458031 |
| [7] | 詹雅琳, 胡文杰, 甄敏, 等. 去蛋白牛骨基质与可吸收胶原膜的磨牙拔牙位点保存效果影像学评价[J]. 北京大学学报(医学版), 2015,47(1):19-26. |
| [8] |
Kim JJ, Schwarz F, Song HY, et al. Ridge preservation of extraction sockets with chronic pathology using Bio-Gide® Collagen with or without collagen membrane: an experimental study in dogs[J]. Clin Oral Implants Res, 2017,28(6):727-733.
doi: 10.1111/clr.12870 pmid: 27194177 |
| [9] |
Wu W, Li B, Liu Y, et al. Effect of multilaminate small intestinal submucosa as a barrier membrane on bone formation in a rabbit mandible defect model[J]. Biomed Res Int, 2018,2018:3270293.
pmid: 30018978 |
| [10] | 吴唯伊, 李博文, 刘玉华, 等. 复层猪小肠黏膜下层可吸收膜的降解性能[J]. 北京大学学报(医学版), 2020,52(3):564-569. |
| [11] |
Eitel F, Klapp F, Jacobson W, et al. Bone regeneration in animals and in man. A contribution to understanding the relative value of animal experiments to human pathophysiology[J]. Arch Orthop Trauma Surg, 1981,99(1):59-64.
pmid: 7316703 |
| [12] |
Lindhe J, Araujo MG, Bufler M, et al. Biphasic alloplastic graft used to preserve the dimension of the edentulous ridge: an experimental study in the dog[J]. Clin Oral Implants Res, 2013,24(10):1158-1163.
pmid: 22804845 |
| [13] |
Naenni N, Sapata V, Bienz SP, et al. Effect of flapless ridge preservation with two different alloplastic materials in sockets with buccal dehiscence defects-volumetric and linear changes[J]. Clin Oral Investig, 2018,22(6):2187-2197.
doi: 10.1007/s00784-017-2309-6 pmid: 29280075 |
| [14] | 詹雅琳, 胡文杰, 徐涛, 等. 罹患重度牙周炎磨牙拔除后应用去蛋白牛骨基质与可吸收胶原膜进行位点保存的组织学研究[J]. 北京大学学报(医学版), 2017,49(1):169-175. |
| [15] |
Benic GI, Thoma DS, Sanz-Martin I, et al. Guided bone regene-ration at zirconia and titanium dental implants: a pilot histological investigation[J]. Clin Oral Implants Res, 2017,28(12):1592-1599.
doi: 10.1111/clr.13030 pmid: 28653343 |
| [16] |
Wang F, Li Q, Wang Z. A comparative study of the effect of Bio-Gide® in combination with concentrated growth factors or bone marrow-derived mesenchymal stem cells in canine sinus grafting[J]. J Oral Pathol Med, 2017,46(7):528-536.
pmid: 27682609 |
| [17] |
Turri A, Elgali I, Vazirisani F, et al. Guided bone regeneration is promoted by the molecular events in the membrane compartment[J]. Biomaterials, 2016,84:167-183.
pmid: 26828682 |
| [18] |
Bouxsein ML, Boyd SK, Christiansen BA, et al. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography[J]. J Bone Miner Res, 2010,25(7):1468-1486.
pmid: 20533309 |
| [19] | Leventis M, Fairbairn P, Mangham C, et al. Bone healing in rabbit calvaria defects using a synthetic bone substitute: A histological and micro-CT comparative study[J]. Materials (Basel), 2018,11(10):1-13. |
| [20] |
Sun Y, Wang CY, Wang ZY, et al. Test in canine extraction site preservations by using mineralized collagen plug with or without membrane[J]. J Biomater Appl, 2016,30(9):1285-1299.
pmid: 26721867 |
| [21] |
Omar O, Dahlin A, Gasser A, et al. Tissue dynamics and rege-nerative outcome in two resorbable non-cross-linked collagen memb-ranes for guided bone regeneration: A preclinical molecular and histological study in vivo[J]. Clin Oral Implants Res, 2018,29(1):7-19.
pmid: 28703398 |
| [1] | 汤莹, 张湧波, 吴丹红, 林炎鸿, 兰风华. 13例先天性双侧输精管缺如不育患者的致病基因突变检测[J]. 北京大学学报(医学版), 2024, 56(5): 763-774. |
| [2] | 王明瑞, 王起, 胡浩, 赖金惠, 唐鑫伟, 万春艳, 许克新, 徐涛. 覆膜金属输尿管支架治疗盆腔脂肪增多症所致肾积水的疗效[J]. 北京大学学报(医学版), 2024, 56(5): 919-922. |
| [3] | 应沂岑,杜毅聪,李志华,张一鸣,李新飞,王冰,张鹏,朱宏建,周利群,杨昆霖,李学松. 机器人辅助腹腔镜下颊黏膜补片输尿管成形术治疗复杂输尿管狭窄[J]. 北京大学学报(医学版), 2024, 56(4): 640-645. |
| [4] | 方杨毅,李强,黄志高,陆敏,洪锴,张树栋. 睾丸鞘膜高分化乳头状间皮肿瘤1例[J]. 北京大学学报(医学版), 2024, 56(4): 741-744. |
| [5] | 侯婉音,董捷. 腹膜透析患者获得性肾囊肿出血3例[J]. 北京大学学报(医学版), 2024, 56(3): 546-550. |
| [6] | 陈晓颖,张一,李雨柯,唐琳,刘玉华. 不同种类聚合物对猪小肠黏膜下层支架仿生矿化的影响[J]. 北京大学学报(医学版), 2024, 56(1): 17-24. |
| [7] | 赵菡,卫彦,张学慧,杨小平,蔡晴,宁成云,徐明明,刘雯雯,黄颖,何颖,郭亚茹,江圣杰,白云洋,吴宇佳,郭雨思,郑晓娜,李文静,邓旭亮. 口腔硬组织修复材料仿生设计制备和临床转化[J]. 北京大学学报(医学版), 2024, 56(1): 4-8. |
| [8] | 段登辉,WANGHom-Lay,王恩博. 可吸收胶原膜在颊侧袋形瓣引导性骨再生手术中的作用: 一项回顾性影像学队列研究[J]. 北京大学学报(医学版), 2023, 55(6): 1097-1104. |
| [9] | 乔婕,芦丽霞,何玉婷,门春翠,楚新新,武蓓,赵慧萍,王梅. 真菌性腹膜透析导管出口感染合并隧道感染1例[J]. 北京大学学报(医学版), 2023, 55(4): 748-754. |
| [10] | 马利加,胡攀攀,刘晓光. 脊柱转移癌伴软脊膜转移1例[J]. 北京大学学报(医学版), 2023, 55(3): 563-566. |
| [11] | 梁丽,李鑫,农琳,董颖,张继新,李东,李挺. 子宫内膜癌微卫星不稳定性分析: 微小微卫星变换的意义[J]. 北京大学学报(医学版), 2023, 55(2): 254-261. |
| [12] | 赖玉梅,李忠武,李欢,吴艳,时云飞,周立新,楼雨彤,崔传亮. 68例肛管直肠黏膜黑色素瘤临床病理特征及预后[J]. 北京大学学报(医学版), 2023, 55(2): 262-269. |
| [13] | 侯卫华,宋书杰,石中月,金木兰. 幽门螺杆菌阴性早期胃癌的临床病理特征[J]. 北京大学学报(医学版), 2023, 55(2): 292-298. |
| [14] | 刘菊梅,梁丽,张继新,戎龙,张梓怡,吴悠,赵旭东,李挺. 411例早期胃癌及癌前病变内镜黏膜下剥离术标本的病理学评估[J]. 北京大学学报(医学版), 2023, 55(2): 299-307. |
| [15] | 宁博涵,张青霞,杨慧,董颖. 伴间质细胞增生、玻璃样变性及索状结构的子宫内膜样腺癌1例[J]. 北京大学学报(医学版), 2023, 55(2): 366-369. |
|
||