北京大学学报(医学版) ›› 2025, Vol. 57 ›› Issue (2): 262-266. doi: 10.19723/j.issn.1671-167X.2025.02.006
大鼠脑创伤半暗带光学相干断层血管造影及微血管密度定量
- 1. 首都医科大学附属北京友谊医院超声医学科,北京 100050
2. 北京大学基础医学院,北京 100191
3. 北京大学人民医院创伤救治中心,创伤救治与神经再生教育部重点实验室(北京大学),国家创伤医学中心 100044
Optical coherence tomography angiography and microvessel density quantification in penumbra after traumatic brain injury in rats
Peng ZHONG1, Xiaodan HU2,3, Zhenzhou WANG3,*( )
)
- 1. Department of Ultrasound, Beijing Friendship Hospital, Capital Medical University, Beijing 100050, China
2. School of Basic Medical Sciences, Peking University, Beijing 100191, China
3. Trauma Center, National Center for Trauma Medicine, Key Laboratory of Trauma and Neural Regeneration, Peking University People's Hospital, Beijing, 100044, China
摘要:
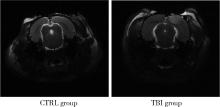
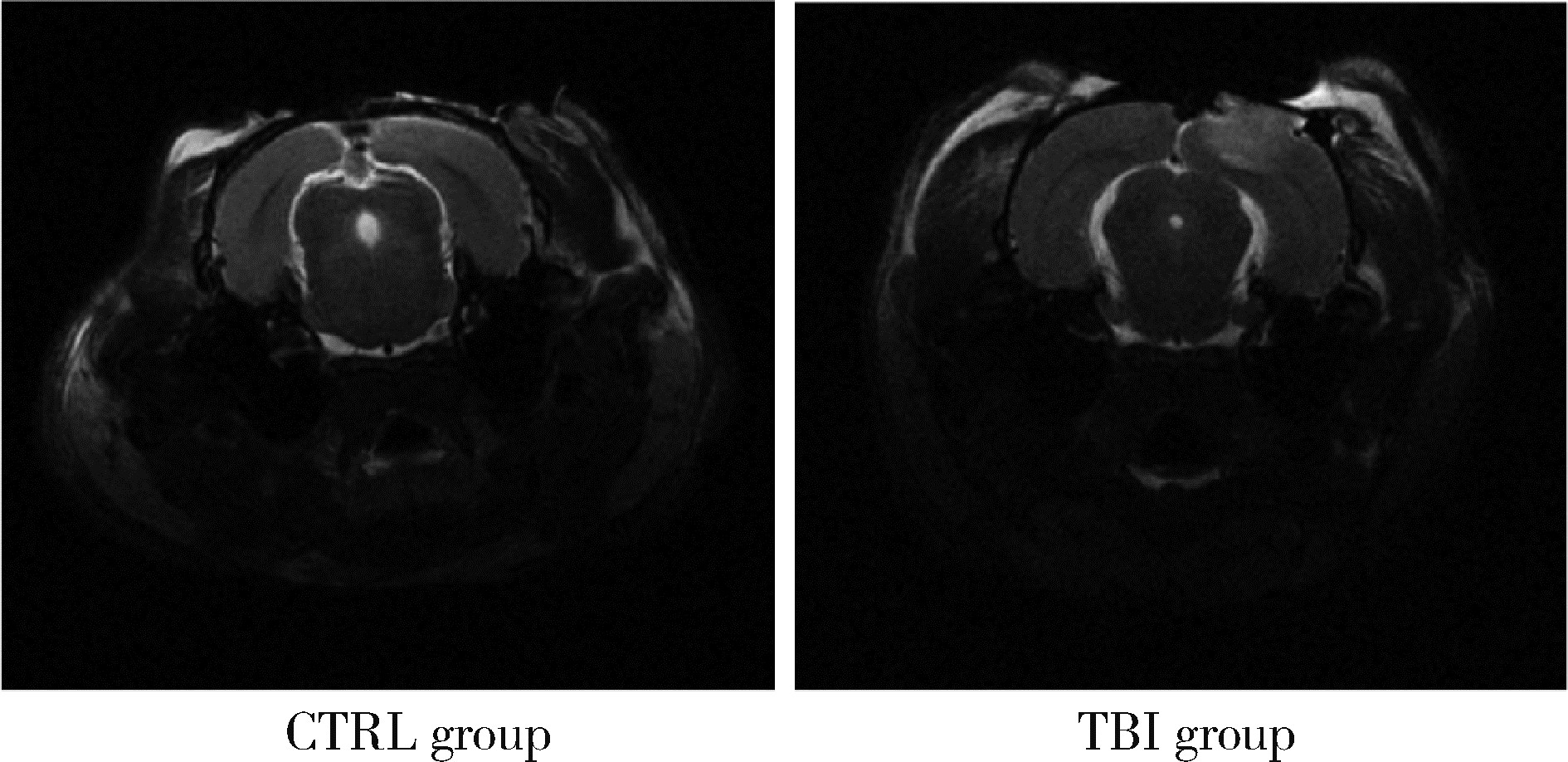
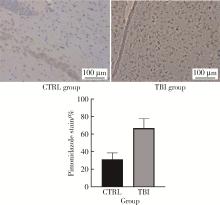
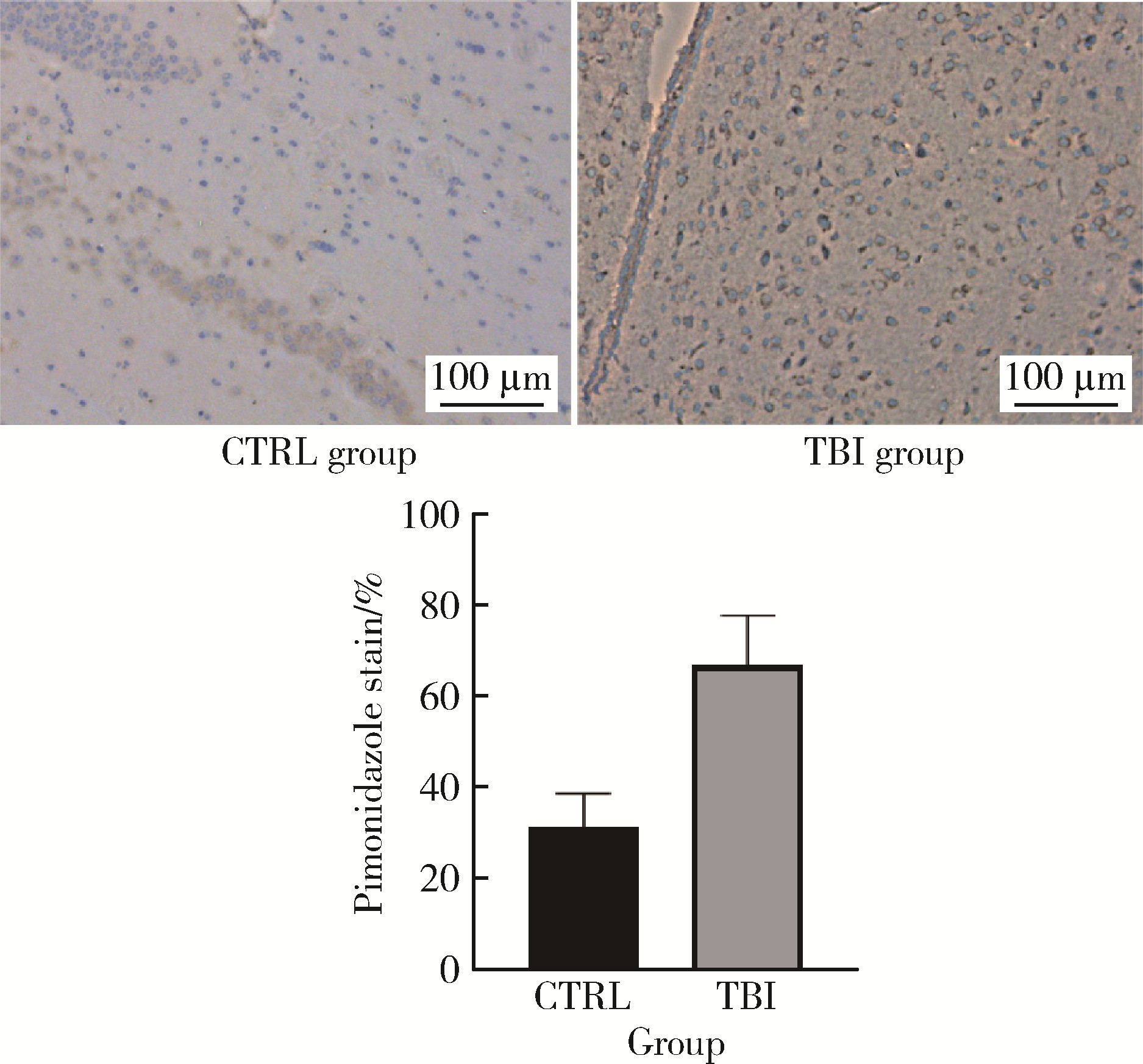
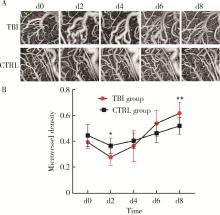
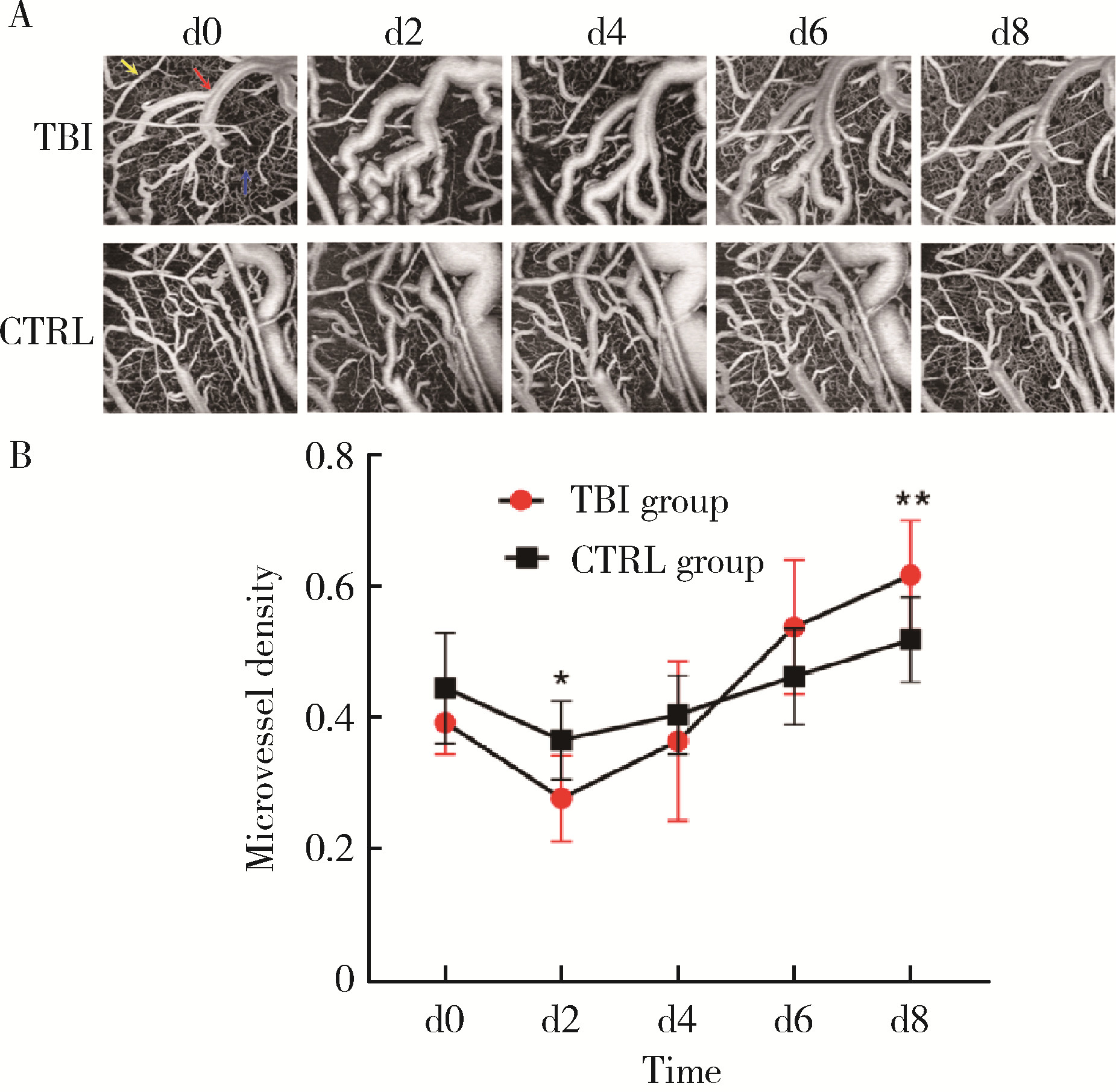
目的: 观察创伤性脑损伤(traumatic brain injury, TBI)大鼠急性期创伤半暗带内微血管损伤与修复的动态变化。方法: 重物坠落致去颅骨骨瓣大鼠TBI后置入封闭式透明颅窗,并设对照组。术后第0、2、4、6、8天经颅窗行光学相干断层血管造影(optical coherence tomography angiography, OCTA)成像,第1天行头部MRI检查,第2天行脑组织哌莫硝唑免疫组织化学检测。结果: MRI T2W1和免疫组织化学检测结果均显示创伤脑组织水肿和缺氧深达脑皮质全层,OCTA示TBI组和对照组两组大鼠术后脑皮质表面静脉均显著扩张迂曲,第8天恢复。TBI组有效灌注微血管密度由第0天的39.38%±4.48%最低降至第2天的27.84%±6.01%,显著低于第0、6、8天(P < 0.05);第8天最高达61.71%±7.69%,显著高于第0、2、4天(P < 0.05);TBI组有效灌注微血管密度第2天显著低于对照组(P=0.021),第8天则显著高于对照组(P=0.030)。结论: OCTA可作为TBI大鼠损伤脑皮质有效灌注微血管成像和计量方法,TBI后创伤半暗带内有效灌注微血管密度降低后逐渐恢复,在第8天显著增加。
中图分类号:
- R651.15
| 1 | Capizzi A , Woo J , Verduzco-Gutierrez M . Traumatic brain injury: An overview of epidemiology, pathophysiology, and medical management[J]. Med Clin North Am, 2020, 104 (2): 213- 238. |
| 2 | Graham DI , Adams JH . Ischaemic brain damage in fatal head injuries[J]. Lancet, 1971, 1 (7693): 265- 266. |
| 3 |
Sahuquillo J , Poca MA , Amoros S . Current aspects of patho-physiology and cell dysfunction after severe head injury[J]. Curr Pharm Des, 2001, 7 (15): 1475- 1503.
doi: 10.2174/1381612013397311 |
| 4 |
Demers-Marcil S , Coles JP . Cerebral metabolic derangements following traumatic brain injury[J]. Curr Opin Anaesthesiol, 2022, 35 (5): 562- 569.
doi: 10.1097/ACO.0000000000001183 |
| 5 |
Hays L , Udy A , Adamides AA , et al. Effects of brain tissue oxygen (PbtO2) guided management on patient outcomes following severe traumatic brain injury: A systematic review and meta-analysis[J]. J Clin Neurosci, 2022, 99, 349- 358.
doi: 10.1016/j.jocn.2022.03.017 |
| 6 |
Reddy L , Murugan D , Mullick M , et al. Recent approaches for angiogenesis in search of successful tissue engineering and rege-neration[J]. Curr Stem Cell Res Ther, 2020, 15 (2): 111- 134.
doi: 10.2174/1574888X14666191104151928 |
| 7 | Bragin DE , Bragina OA , Kameneva MV , et al. Resuscitation with drag reducing polymers after traumatic brain injury with hemor-rhagic shock reduces microthrombosis and oxidative stress[J]. Adv Exp Med Biol, 2020, 1232, 39- 45. |
| 8 |
de Carlo TE , Romano A , Waheed NK , et al. A review of optical coherence tomography angiography (OCTA)[J]. Int J Retina Vitreous, 2015, 1, 5.
doi: 10.1186/s40942-015-0005-8 |
| 9 |
Feeney DM , Boyeson MG , Linn RT , et al. Responses to cortical injury: Ⅰ. Methodology and local effects of contusions in the rat[J]. Brain Res, 1981, 211 (1): 67- 77.
doi: 10.1016/0006-8993(81)90067-6 |
| 10 |
Liu X , Huang Z , Wang Z , et al. A deep learning based pipeline for optical coherence tomography angiography[J]. J Biophotonics, 2019, 12 (10): e201900008.
doi: 10.1002/jbio.201900008 |
| 11 |
Wang Z , Liu J , Liu X , et al. Perfusion microvessel density in the cerebral cortex of septic rats is negatively correlated with endothe-lial microparticles in circulating plasma[J]. Metab Brain Dis, 2021, 36 (5): 1029- 1036.
doi: 10.1007/s11011-021-00702-x |
| 12 |
Veenith TV , Carter EL , Geeraerts T , et al. Pathophysiologic mechanisms of cerebral ischemia and diffusion hypoxia in traumatic brain injury[J]. JAMA Neurol, 2016, 73 (5): 542- 550.
doi: 10.1001/jamaneurol.2016.0091 |
| 13 |
Sandsmark DK , Bashir A , Wellington CL , et al. Cerebral microvascular injury: A potentially treatable endophenotype of traumatic brain injury-induced neurodegeneration[J]. Neuron, 2019, 103 (3): 367- 379.
doi: 10.1016/j.neuron.2019.06.002 |
| 14 | Kenney K , Amyot F , Haber M , et al. Cerebral vascular injury in traumatic brain injury[J]. Exp Neurol, 2016, 275 (Pt 3): 353- 366. |
| 15 | Manglani M , McGavern DB . Intravital imaging of neuroimmune interactions through a thinned skull[J]. Curr Protoc Immunol, 2018, 120, 24.2.1- 24.2.12. |
| 16 |
Hattori R , Komiyama T . Longitudinal two-photon calcium imaging with ultra-large cranial window for head-fixed mice[J]. STAR Protoc, 2022, 3 (2): 101343.
doi: 10.1016/j.xpro.2022.101343 |
| 17 | Qin D , Wang J , Le A , et al. Traumatic brain injury: Ultrastructural features in neuronal ferroptosis, glial cell activation and polarization, and blood-brain barrier breakdown[J]. Cells, 2021, 10 (5): 1009. |
| 18 | Grutzendler J , Murikinati S , Hiner B , et al. Angiophagy prevents early embolus washout but recanalizes microvessels through embolus extravasation[J]. Sci Transl Med, 2014, 6 (226): 226ra31. |
| 19 |
van der Wijk AE , Georgakopoulou T , Majolée J , et al. Microembolus clearance through angiophagy is an auxiliary mechanism preserving tissue perfusion in the rat brain[J]. Acta Neuropathol Commun, 2020, 8 (1): 195.
doi: 10.1186/s40478-020-01071-9 |
| 20 |
van der Wijk AE , Lachkar N , de Vos J , et al. Extravasation of microspheres in a rat model of silent brain infarcts[J]. Stroke, 2019, 50 (6): 1590- 1594.
doi: 10.1161/STROKEAHA.119.024975 |
| 21 | Park E , Bell JD , Siddiq IP , et al. An analysis of regional microvascular loss and recovery following two grades of fluid percussion trauma: A role for hypoxia-inducible factors in traumatic brain injury[J]. J Cereb Blood Flow Metab, 2009, 29 (3): 575- 584. |
| [1] | 叶珊,金萍萍,张楠,邬海博,石林,赵强,杨坤,袁慧书,樊东升. 肌萎缩侧索硬化患者认知功能改变与脑皮层厚度分析[J]. 北京大学学报(医学版), 2022, 54(6): 1158-1162. |
| [2] | 姜茜, 姜玉武, 王静敏, 秦炯, 吴希如 . 一种改进的大鼠皮层神经元原代培养方法及其性质鉴定[J]. 北京大学学报(医学版), 2009, 41(2): 212-216. |
| [3] | 张玉稚, 潘虹, 王汉森, 包新华, 吴希如. 发育中Wistar大鼠大脑皮层甲基化结合蛋白-2基因表达变化[J]. 北京大学学报(医学版), 2004, 36(4): 379-382. |
| [4] | 曹海燕, 姜玉武, 何其华, 陈跃, 袁兰, 吴希如. 细胞内游离Ca2+在发育中大鼠皮层神经元无镁诱导惊厥性损伤中的作用[J]. 北京大学学报(医学版), 2003, 35(5): 466-470. |
|
||