北京大学学报(医学版) ›› 2026, Vol. 58 ›› Issue (1): 22-29. doi: 10.19723/j.issn.1671-167X.2026.01.003
细胞膜囊泡递送靶向肿瘤坏死因子-α的小干扰RNA对牙髓干细胞的抗炎作用
高若凡, 马天宇, 王润楷, 殷雨辰, 李芮迪, 王丹丹*( ), 夏斌*(
), 夏斌*( )
)
- 北京大学口腔医学院·口腔医院儿童口腔科, 国家口腔医学中心, 国家口腔疾病临床医学研究中心, 口腔生物材料和数字诊疗装备国家工程研究中心, 北京 100081
Anti-inflammatory effects of cell membrane vesicle-mediated delivery of small interfering RNA targeting tumor necrosis factor-α on dental pulp stem cells
Ruofan GAO, Tianyu MA, Runkai WANG, Yuchen YIN, Ruidi LI, Dandan WANG*( ), Bin XIA*(
), Bin XIA*( )
)
- Department of Pediatric Dentistry, Peking University School and Hospital of Stomatology & National Center of Stomatology & National Clinical Research Center for Oral Diseases & National Engineering Laboratory for Digital and Material Technology of Stomatology, Beijing 100081, China
摘要:
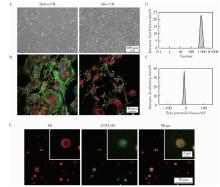
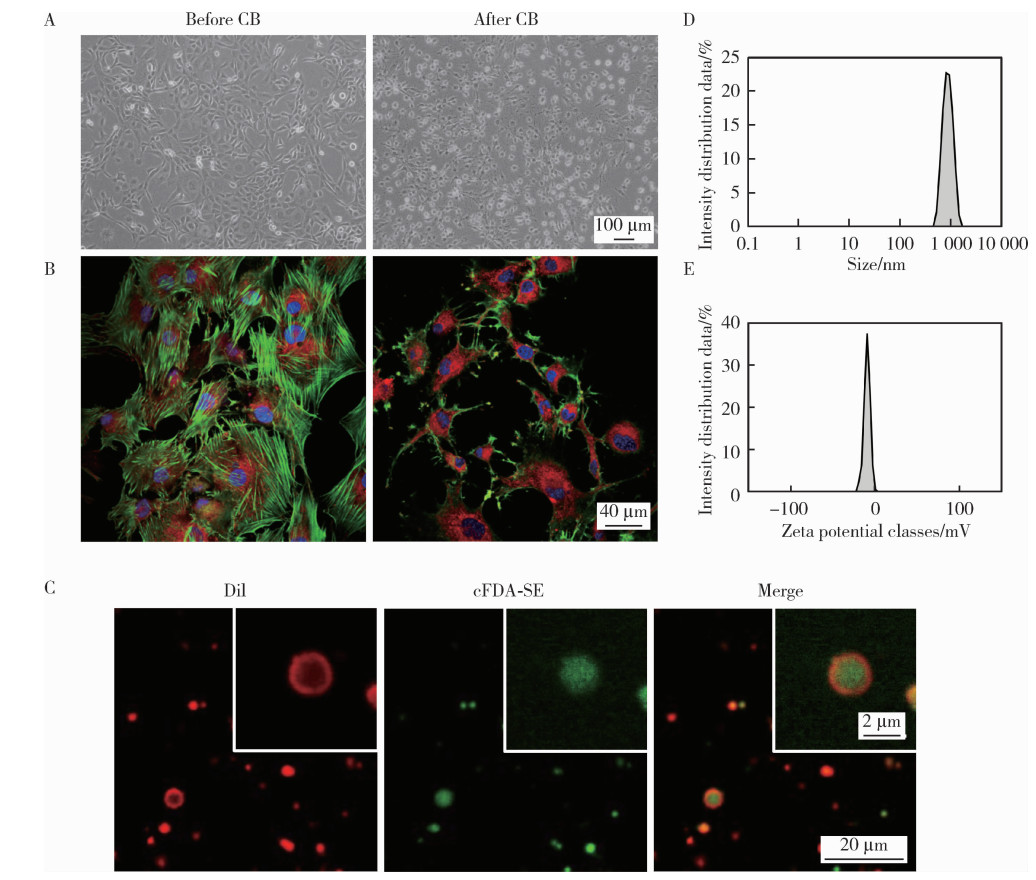
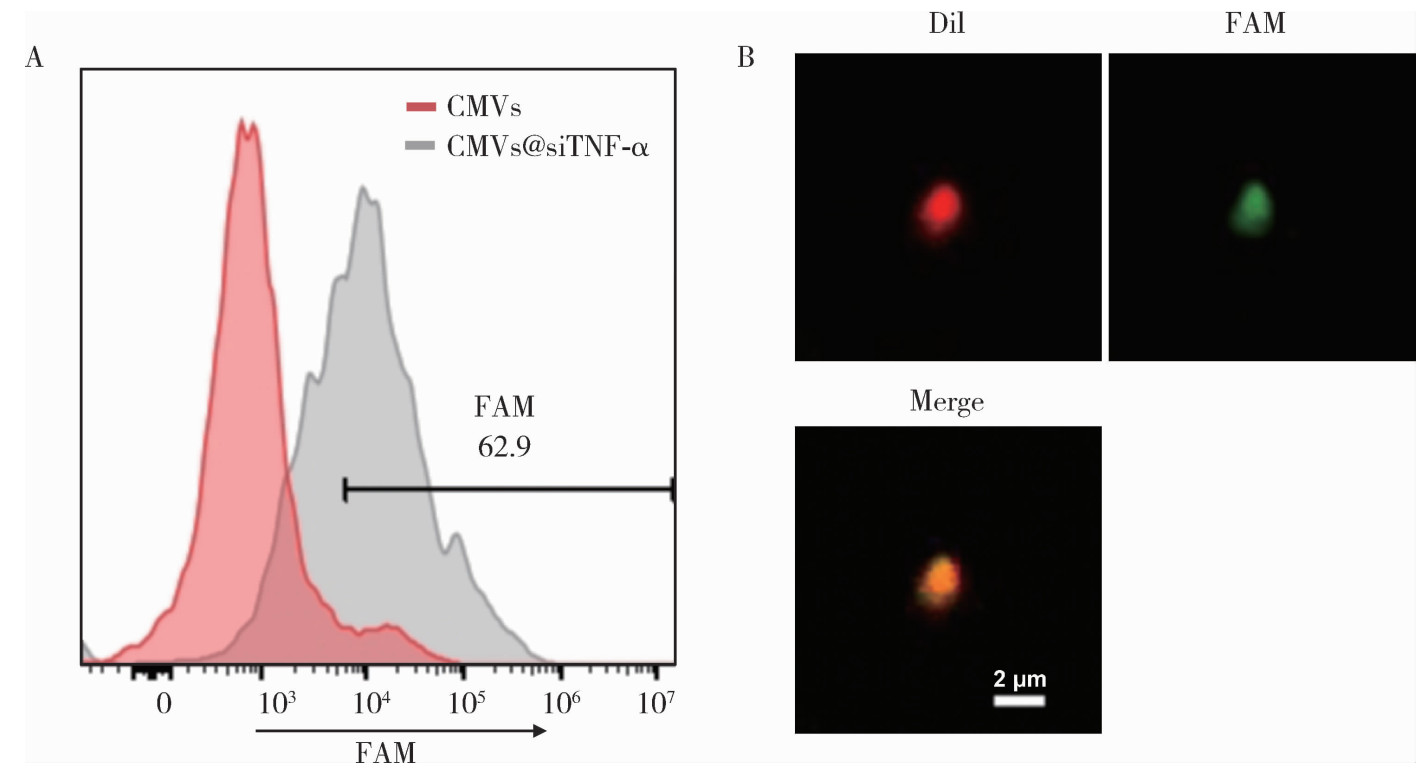
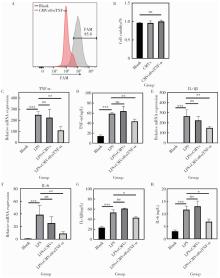
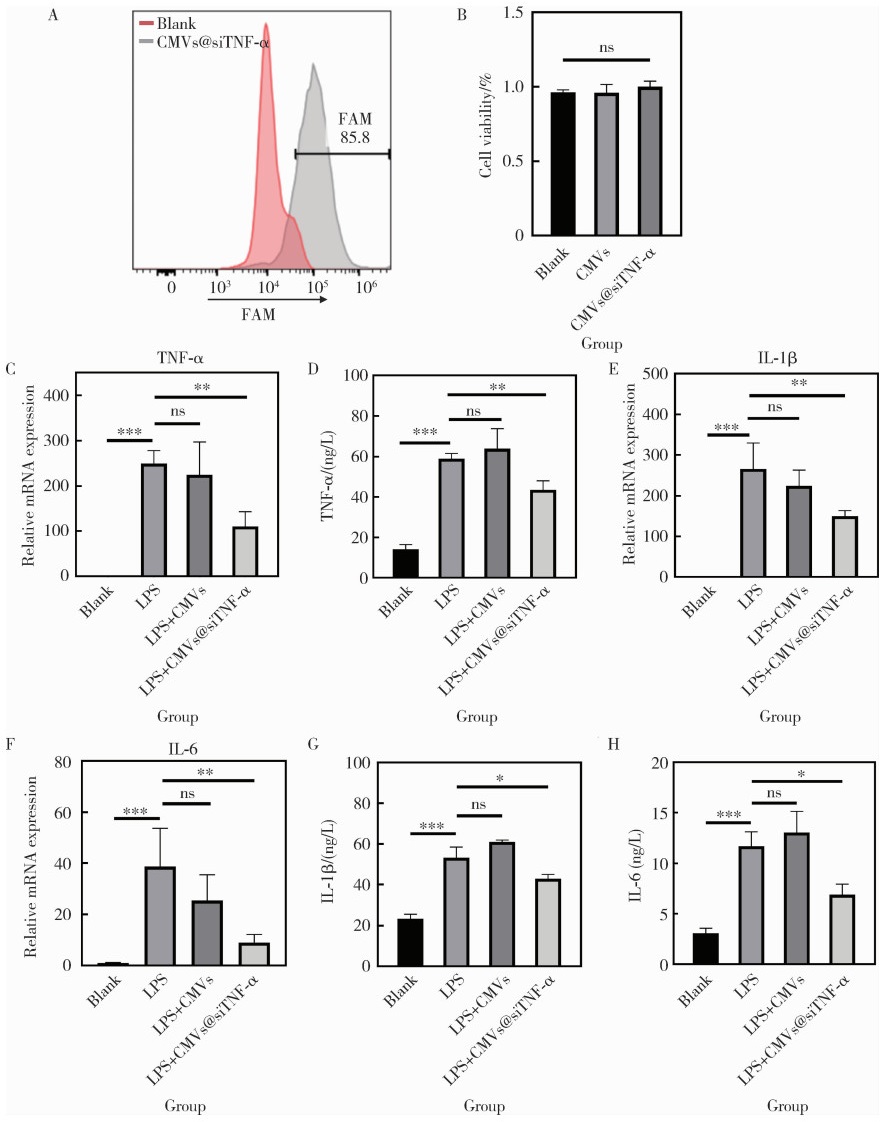
目的: 探索细胞膜囊泡(cell membrane vesicles, CMVs)作为广泛适用的基因沉默工具小干扰RNA(small interfering RNA, siRNA)递送平台的可行性, 并以脂多糖(lipopolysaccharide, LPS)诱导的人牙髓干细胞(dental pulp stem cells, DPSCs)炎症模型验证其应用效果。方法: 采用细胞松弛素B处理3T3细胞制备CMVs, 并通过共聚焦显微镜、动态光散射检测CMVs理化性质。利用洋地黄皂苷透化CMVs将靶向肿瘤坏死因子-α(tumor necrosis factor-α, TNF-α)的siRNA(siTNF-α)负载至CMVs中, 构建CMVs@siTNF-α。通过流式细胞术和共聚焦显微镜检测siRNA负载与胞内转运效率。以LPS(1 mg/L)刺激DPSCs建立炎症模型, 并与CMVs或CMVs@siTNF-α共培养。采用细胞活力检测试剂盒(cell counting kit-8, CCK-8)检测细胞毒性, 实时荧光定量聚合酶链反应(quantitative real-time polymerase chain reaction, qRT-PCR)和酶联免疫吸附试验(enzyme linked immunosorbent assay, ELISA)检测TNF-α、白介素(interleukin, IL)-1β和IL-6的表达与分泌。结果: CMVs呈球形, 由细胞膜及细胞质构成, 平均粒径为903 nm, 表面电位为-9.39 mV。流式细胞术结果显示, 负载荧光素酰胺(fluorescein amidite, FAM)标记siRNA(FAM-siRNA)后CMVs荧光强度升高; DPSCs加入CMVs@FAM-siTNF-α培养24 h后, 荧光信号增强。CCK-8检测结果显示CMVs及CMVs@siTNF-α对DPSCs无明显毒性。LPS处理显著上调DPSCs中TNF-α、IL-1β和IL-6的表达, 与LPS组相比, CMVs@siTNF-α下调TNF-α mRNA及其蛋白表达水平, 并抑制IL-1β和IL-6的转录与分泌。单独CMVs处理无明显差异。结论: CMVs可作为一种低毒性的siRNA递送平台, 在体外炎症模型中实现TNF-α沉默及下游炎症反应抑制, 具有进一步研究和应用的潜力。
中图分类号:
- R78
| 1 |
doi: 10.1038/s41587-023-02105-y |
| 2 |
doi: 10.1016/j.ejphar.2021.174178 |
| 3 |
doi: 10.1016/j.ymthe.2024.01.005 |
| 4 |
doi: 10.1021/acs.accounts.9b00368 |
| 5 |
doi: 10.1038/s41573-024-00912-9 |
| 6 |
doi: 10.1093/nar/gky1239 |
| 7 |
doi: 10.1093/nar/gkx960 |
| 8 |
doi: 10.1093/nar/gkac539 |
| 9 |
doi: 10.1186/s12964-023-01103-6 |
| 10 |
doi: 10.1016/j.addr.2021.03.005 |
| 11 |
doi: 10.1002/advs.202101562 |
| 12 |
|
| 13 |
doi: 10.1016/j.jconrel.2018.02.031 |
| 14 |
doi: 10.3390/mi10110750 |
| 15 |
doi: 10.3390/ijms26094325 |
| 16 |
doi: 10.1111/iej.14108 |
| 17 |
doi: 10.1186/s12903-022-02161-x |
| 18 |
doi: 10.1177/154405910508401105 |
| 19 |
doi: 10.1186/s12974-025-03356-z |
| 20 |
doi: 10.3389/fcell.2025.1511577 |
| 21 |
doi: 10.1021/ja044605x |
| 22 |
doi: 10.1093/nar/gkaa670 |
| 23 |
doi: 10.1016/j.omtm.2025.101436 |
| 24 |
doi: 10.1021/acsami.5b05065 |
| 25 |
doi: 10.1042/BCJ20210584 |
| 26 |
doi: 10.1038/s41413-024-00325-9 |
| 27 |
doi: 10.1080/20013078.2017.1265291 |
| 28 |
doi: 10.1021/bc500291r |
| 29 |
doi: 10.1111/iej.14078 |
| 30 |
doi: 10.1186/s12974-023-02747-4 |
| 31 |
doi: 10.1136/ard-2022-222605 |
| 32 |
doi: 10.1038/s41577-024-01008-6 |
| 33 |
doi: 10.1021/acsnano.1c03800 |
| [1] | 李梦迪, 雷蕾, 刘中宁, 李健, 姜婷. siRNA沉默NLK基因促进神经化组织工程骨再生[J]. 北京大学学报(医学版), 2025, 57(2): 227-236. |
| [2] | 叶雨阳,岳林,邹晓英,王晓燕. 成牙本质方向分化牙髓干细胞外泌体形态及微小RNA表达谱特征[J]. 北京大学学报(医学版), 2023, 55(4): 689-696. |
| [3] | 娄雪,廖莉,李兴珺,王楠,刘爽,崔若玫,徐健. 类风湿关节炎患者外周血TWEAK基因启动子区甲基化状态及其表达[J]. 北京大学学报(医学版), 2021, 53(6): 1020-1025. |
| [4] | 胡永玮,刘蕊,罗莉. 慢性多灶性骨髓炎1例及文献回顾[J]. 北京大学学报(医学版), 2020, 52(6): 1140-1145. |
| [5] | 高晓敏,邹晓英,岳林. 根尖牙乳头干细胞摄取外泌体的介导途径[J]. 北京大学学报(医学版), 2020, 52(1): 43-50. |
| [6] | 谢静,赵玉鸣,饶南荃,汪晓彤,方滕姣子,李晓霞,翟越,李静芝,葛立宏,王媛媛. 3种口腔颌面部来源的间充质干细胞成血管内皮分化潜能的比较研究[J]. 北京大学学报(医学版), 2019, 51(5): 900-906. |
| [7] | 汪晓彤,饶南荃,方腾姣子,赵玉鸣,葛立宏. 乳牙牙髓干细胞CD146阳性/阴性细胞亚群生物学特性的比较[J]. 北京大学学报(医学版), 2018, 50(2): 284-292. |
| [8] | 贾维茜,赵玉鸣,葛立宏. 人重组转化生长因子β1促进牙髓干细胞的增殖和矿化[J]. 北京大学学报(医学版), 2017, 49(4): 680-681. |
| [9] | 王艳飞, 贾新未, 赵文萍, 王凤娟, 刘亚宁, 张芳, 张丽敏, 王鸿超. 辛伐他汀后适应对缺血再灌注损伤大鼠TNF-α及NF-κB的影响[J]. 北京大学学报(医学版), 2014, 46(6): 990-992. |
| [10] | 余日月*, 曾百进. 重组人肿瘤坏死因子α对人脂肪基质细胞体外成骨向分化的影响[J]. 北京大学学报(医学版), 2012, 44(3): 475-480. |
| [11] | 王秀茹*, 苏茵, 安媛, 周云杉, 张晓盈, 段天骄, 朱佳鑫, 李小峰, 王彩虹, 王莉枝, 王永福, 杨荣, 王国春, 卢昕, 朱平, 陈丽娜, 王轶, . 我国类风湿关节炎患者应用肿瘤坏死因子抑制剂现况调查[J]. 北京大学学报(医学版), 2012, 44(2): 182-187. |
| [12] | 梁建涛, 王振宇. 脑动脉狭窄患者血清中炎性因子干扰素-γ、白细胞介素-6和肿瘤坏死因子-α的水平分析[J]. 北京大学学报(医学版), 2011, 43(6): 837-840. |
| [13] | 刘洋, 金建秋, 袁振芳, 刘晓松, 曹婕, 郭晓蕙, 刘宏伟. 2型糖尿病伴口腔扁平苔藓患者唾液白细胞介素-6和肿瘤坏死因子-α水平[J]. 北京大学学报(医学版), 2011, 43(4): 596-599. |
| [14] | 曾百进, 余日月, 周永胜, 徐军, 倪永伟, 刘云松, 许永伟. rhTNF-α对成骨向分化前后的人脂肪基质细胞分泌血管生成相关生长因子的影响[J]. 北京大学学报(医学版), 2009, 41(5): 565-570. |
| [15] | 孙晓军, 孟焕新, 陈智滨, 徐莉, 张立, 释栋, 冯向辉. 侵袭性牙周炎患者血浆白细胞介素-1β和肿瘤坏死因子-α的检测[J]. 北京大学学报(医学版), 2008, 40(1): 24-27. |
|
||