北京大学学报(医学版) ›› 2019, Vol. 51 ›› Issue (1): 9-15. doi: 10.19723/j.issn.1671-167X.2019.01.003
先天性缺牙患者中BMP2基因突变检测及功能分析
- 北京大学口腔医学院·口腔医院,修复科 国家口腔疾病临床研究中心 口腔数字化医疗技术和材料国家工程实验室 口腔数字医学北京市重点实验室,北京 100081
Detection and functional analysis of BMP2 gene mutation in patients with tooth agenesis
Hao WANG,Yang LIU,Hao-chen LIU,Dong HAN( ),Hai-lan FENG
),Hai-lan FENG
- Department of Prosthodontics, Peking University School and Hospital of Stomatology & National Clinical Research Center for Oral Diseases & National Engineering Laboratory for Digital and Material Technology of Stomatology & Beijing Key Laboratory of Digital Stomatology, Beijing 100081, China
摘要:
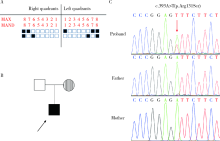
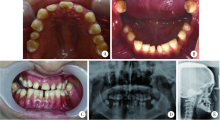
目的:在先天性缺牙患者中检测骨形态发生蛋白2(bone morphogenetic protein 2, BMP2)基因突变, 记录BMP2基因突变相关的临床表型, 并进行突变功能分析以评估突变致病性。方法:选取18名先天性缺牙患者, 进行病史采集、临床检查、X线检查, 采集血液样本提取DNA, 全外显子测序,选取和牙颌面发育相关或骨骼系统遗传性疾病相关的基因进行分析,筛选可能致病的基因突变, 选取可能有功能影响的BMP2突变, 分析患者临床表现,进行突变功能试验。构建野生型和突变型BMP2质粒转染人胚胎肾293T细胞, 激光扫描共聚焦显微镜下观察蛋白质在细胞内分布, 蛋白质印迹实验检测突变型BMP2磷酸化激活下游SMAD1/5/9分子(SMAD family member 1/5/9, SMAD1/5/9)的情况。结果:在1例先天性缺牙患者中检测到可能有功能影响的BMP2突变NM_001200.3:c.393A>T(p.Arg131Ser), rs140417301, 患者父母不携带此突变,患者父亲牙齿正常,母亲先天缺失1颗前磨牙,父母上颌形态均正常。患者同时具有上颌骨前后向及水平向发育不足、上腭形态发育异常, 及继发的错颌畸形, 同时对腰椎、髋部骨的X线检查提示骨密度减低。功能试验显示该突变对骨形态发生蛋白(bone morphogenetic protein, BMP)通路活性有影响,该突变导致BMP2蛋白质磷酸化激活下游SMAD1/5/9蛋白质的能力减弱(3次重复实验分别下降32%、22%、27%), 结合家族共分离等判断该突变为“可能致病的”。结论:BMP2突变c.393A>T(p.Arg131Ser)对BMP通路活性具有一定影响,可使得突变型BMP2蛋白质对于下游SMAD1/5/9蛋白质激活能力减弱,在临床上可能导致先天性缺牙、上颌骨发育不足、上腭发育异常、错颌畸形及骨密度降低。
中图分类号:
- R783.4
| [1] |
Wozney JM . The bone morphogenetic protein family and osteoge-nesis[J]. Mol Reprod Dev, 1992,32(2):160-167.
doi: 10.1002/mrd.1080320212 pmid: 1637554 |
| [2] |
Zhang YD, Chen Z, Song YQ , et al. Making a tooth: growth factors, transcription factors, and stem cells[J]. Cell Res, 2005,15(5):301-316.
doi: 10.1038/sj.cr.7290299 pmid: 15916718 |
| [3] |
Nishinakamura R, Sakaguchi M . BMP signaling and its modifiers in kidney development[J]. Pediatr Nephrol, 2014,29(4):681-686.
doi: 10.1007/s00467-013-2671-9 pmid: 24217785 |
| [4] |
Neubuser A, Peters H, Balling R , et al. Antagonistic interactions between FGF and BMP signaling pathways: a mechanism for positioning the sites of tooth formation[J]. Cell, 1997,90(2):247-255.
doi: 10.1016/S0092-8674(00)80333-5 pmid: 9244299 |
| [5] |
Yuan G, Yang G, Zheng Y , et al. The non-canonical BMP and Wnt/beta-catenin signaling pathways orchestrate early tooth deve-lopment[J]. Development, 2015,142(1):128-139.
doi: 10.1242/dev.117887 pmid: 25428587 |
| [6] |
Zhao M, Harris SE, Horn D , et al. Bone morphogenetic protein receptor signaling is necessary for normal murine postnatal bone formation[J]. J Cell Biol, 2002,157(6):1049-1060.
doi: 10.1083/jcb.200109012 pmid: 12058020 |
| [7] | de Coster PJ, Marks LA, Martens LC , et al. Dental agenesis: genetic and clinical perspectives[J]. J Oral Pathol Med, 2009,38(1):1-17. |
| [8] |
Yin W, Bian Z . The gene network underlying hypodontia[J]. J Dent Res, 2015,94(7):878-885.
doi: 10.1177/0022034515583999 pmid: 25910507 |
| [9] |
Kantaputra P, Sripathomsawat W . WNT10A and isolated hypodontia[J]. Am J Med Genet A, 2011,155(5):1119-1122.
doi: 10.1002/ajmg.a.33840 pmid: 21484994 |
| [10] |
Wong S, Liu H, Bai B , et al. Novel missense mutations in the AXIN2 gene associated with non-syndromic oligodontia[J]. Arch Oral Biol, 2014,59(3):349-353.
doi: 10.1016/j.archoralbio.2013.12.009 pmid: 24581859 |
| [11] |
Mues GI, Griggs R, Hartung AJ , et al. From ectodermal dysplasia to selective tooth agenesis[J]. Am J Med Genet A, 2009,149(9):2037-2041.
doi: 10.1002/ajmg.a.32801 pmid: 19504606 |
| [12] |
Wong S, Liu H, Han D , et al. A novel non-stop mutation in MSX1 causing autosomal dominant non-syndromic oligodontia[J]. Mutagenesis, 2014,29(5):319-323.
doi: 10.1093/mutage/geu019 pmid: 24914010 |
| [13] |
Stockton DW, Das P, Goldenberg M , et al. Mutation of PAX9 is associated with oligodontia[J]. Nat Genet, 2000,24(1):18-19.
doi: 10.1038/71634 pmid: 10615120 |
| [14] |
Yu P, Yang W, Han D , et al. Mutations in WNT10B are identified in individuals with oligodontia[J]. Am J Hum Genet, 2016,99(1):195-201.
doi: 10.1016/j.ajhg.2016.05.012 pmid: 27321946 |
| [15] |
Liu X, Gao L, Zhao A , et al. Identification of duplication downstream of BMP2 in a Chinese family with brachydactyly type A2 (BDA2)[J]. PLoS One, 2014,9(4):e94201.
doi: 10.1371/journal.pone.0094201 pmid: 3978006 |
| [16] |
Styrkarsdottir U, Cazier JB, Kong A , et al. Linkage of osteoporosis to chromosome 20p12 and association to BMP2[J]. PLoS Biol, 2003,1(3):E69.
doi: 10.1371/journal.pbio.0000069 pmid: 14691541 |
| [17] |
Sahoo T, Theisen A, Sanchez-Lara PA , et al. Microdeletion 20p12.3 involving BMP2 contributes to syndromic forms of cleft palate[J]. Am J Med Genet A, 2011,155(7):1646-1653.
doi: 10.1002/ajmg.a.34063 pmid: 3121907 |
| [18] |
Williams ES, Uhas KA, Bunke BP , et al. Cleft palate in a multigenerational family with a microdeletion of 20p12.3 involving BMP2[J]. Am J Med Genet A, 2012,158(10):2616-2620.
doi: 10.1002/ajmg.a.35594 pmid: 22965927 |
| [19] |
Mu Y, Xu Z, Contreras CI , et al. Phenotype characterization and sequence analysis of BMP2 and BMP4 variants in two Mexican families with oligodontia[J]. Genet Mol Res, 2012,11(4):4110-4120.
doi: 10.4238/2012.September.25.5 pmid: 23079991 |
| [20] | 邹川, 高清平, Bakrv HH, , 等. 单纯性先天缺牙患者BMP2/BMP4基因位点分析[J]. 上海口腔医学, 2015,24(1):83-88. |
| [21] |
Lu Y, Qian Y, Zhang J , et al Genetic variants of BMP2 and their association with the risk of non-syndromic tooth agenesis[J]. PLoS One, 2016,11(6):e0158273.
doi: 10.1371/journal.pone.0158273 pmid: 27362534 |
| [22] |
Prasad MK, Geoffroy V, Vicaire S , et al. A targeted next-generation sequencing assay for the molecular diagnosis of genetic disorders with orodental involvement[J]. J Med Genet, 2016,53(2):98-110.
doi: 10.1136/jmedgenet-2015-103302 pmid: 4752661 |
| [23] |
Clark GR, Duncan EL . The genetics of osteoporosis[J]. Br Med Bull, 2015,113(1):73-81.
doi: 10.1093/bmb/ldu042 |
| [24] |
Richards JB, Zheng HF, Spector TD . Genetics of osteoporosis from genome-wide association studies: advances and challenges[J]. Nat Rev Genet, 2012,13(8):576-588.
doi: 10.1038/nrg3228 pmid: 22805710 |
| [25] |
Hsu YH, Kiel DP . Clinical review: Genome-wide association studies of skeletal phenotypes: what we have learned and where we are headed[J]. J Clin Endocrinol Metab, 2012,97(10):E1958-E1977.
doi: 10.1210/jc.2012-1890 pmid: 22965941 |
| [26] |
Richards S, Aziz N, Bale S , et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology[J]. Genet Med, 2015,17(5):405-424.
doi: 10.1038/gim.2015.30 pmid: 4544753 |
| [27] |
Tan TY, Gonzaga-Jauregui C, Bhoj EJ , et al. Monoallelic BMP2 variants predicted to result in haploinsufficiency cause craniofacial, skeletal, and cardiac features overlapping those of 20p12 deletions[J]. Am J Hum Genet, 2017,101(6):985-994.
doi: 10.1016/j.ajhg.2017.10.006 pmid: 29198724 |
| [28] |
Timberlake AT, Choi J, Zaidi S , et al. Two locus inheritance of non-syndromic midline craniosynostosis via rare SMAD6 and common BMP2 alleles[J]. Elife, 2016,5(4 suppl):1.
doi: 10.7554/eLife.20125 pmid: 5045293 |
| [29] |
Radio FC, Majore S, Aurizi C , et al. Hereditary hemochromatosis type 1 phenotype modifiers in Italian patients. The controversial role of variants in HAMP, BMP2, FTL and SLC40A1 genes[J]. Blood Cells Mol Dis, 2015,55(1):71-75.
doi: 10.1016/j.bcmd.2015.04.001 pmid: 25976471 |
| [30] |
Breitfeld J, Martens S, Klammt J , et al. Genetic analyses of bone morphogenetic protein 2, 4 and 7 in congenital combined pituitary hormone deficiency[J]. BMC Endocr Disord, 2013,13:56.
doi: 10.1186/1472-6823-13-56 pmid: 4175098 |
| [31] |
Xu XL, Lou J, Tang T , et al. Evaluation of different scaffolds for BMP2 genetic orthopedic tissue engineering[J]. J Biomed Mater Res B Appl Biomater, 2005,75(2):289-303.
doi: 10.1002/jbm.b.30299 pmid: 16025445 |
| [1] | 吴君怡,余淼,孙仕晨,樊壮壮,郑静蕾,张刘陶,冯海兰,刘洋,韩冬. 少汗性外胚层发育不良患者EDA基因突变检测及表型分析[J]. 北京大学学报(医学版), 2021, 53(1): 24-33. |
|
||