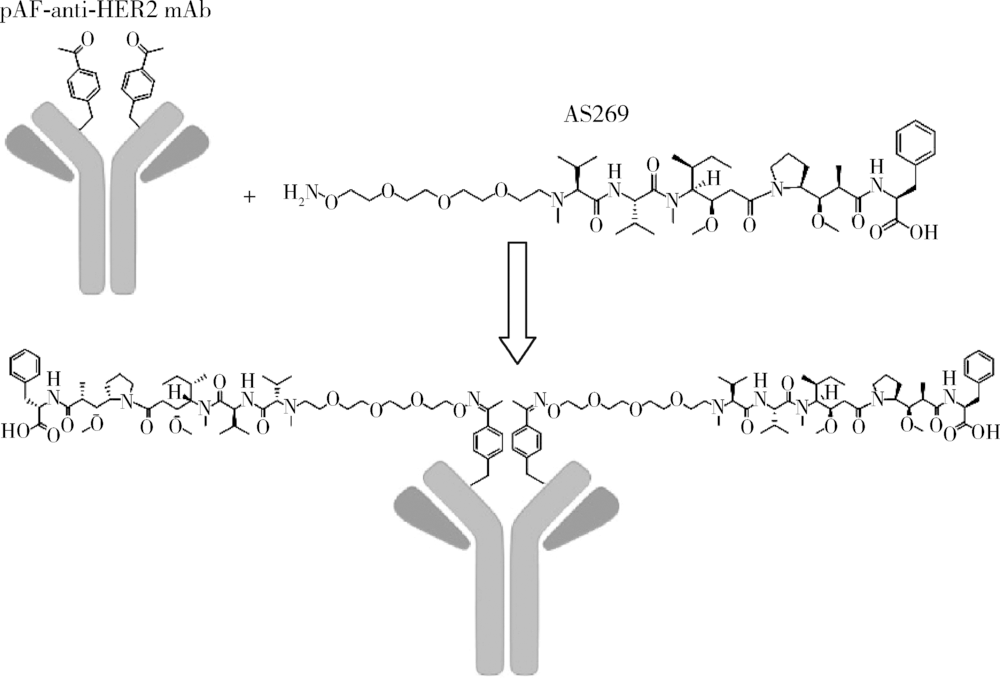
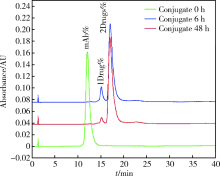
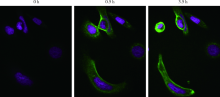
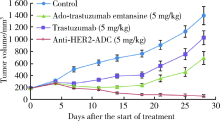
北京大学学报(医学版) ›› 2019, Vol. 51 ›› Issue (5): 797-804. doi: 10.19723/j.issn.1671-167X.2019.05.002
非天然氨基酸定点偶联抗人类表皮生长因子受体2-抗体偶联药物的药理学活性
- 1. 浙江新码生物医药有限公司,浙江绍兴 312000
2. 北京大学药学院化学生物学系,北京 100871
Pharmacological effects of site specific conjugated anti-human epidermal growth factor receptor 2-antibody drug conjugate using unnatural amino acid technology
Xue-jun LIANG1,Li-ying GONG1,Fei ZHOU1,De-min ZHOU2,Jing-jing ZHU1,△( )
)
- 1. Zhejiang NovoCodex Biopharmaceuticals Company Limited, Shaoxing, Zhejiang 312000, China
2. Department of Chemical Biology, School of Pharmaceutical Sciences, Peking University, Beijing 100871, China
摘要:
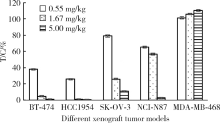
目的:考察用非天然氨基酸定点偶联技术获得的均一性良好的抗人类表皮生长因子受体2(human epidermal growth factor receptor 2, HER2)-抗体偶联药物(antibody drug conjugate, ADC), 对9种不同HER2表达量的肿瘤细胞的增殖抑制活性,以及对5种异体移植的肿瘤小鼠模型的肿瘤生长抑制效果。方法:用QIFI试剂盒通过流式细胞仪检测HER2在BT-474、Calu-3、MCF-7、MDA-MB-231、MDA-MB-468、SK-BR-3、SK-OV-3、HCC1954、NCI-N87共9种肿瘤细胞中的表达。对9种肿瘤细胞进行培养,铺板过夜后分别加入梯度稀释的抗HER2-ADC、曲妥珠单抗-美坦新偶联物、AS269、pAF-AS269、紫杉醇5种药物,然后培养72 h或96 h,检测这5种药物对肿瘤细胞的增殖抑制活性。选择HER2阳性肿瘤细胞HCC1954、BT-474、SK-OV-3、NCI-N87和HER2阴性肿瘤细胞MDA-MB-468,分别接种到5~6周龄的BALB/c裸小鼠身上,待肿瘤长到一定体积后,分别注射抗HER2-ADC、曲妥珠单抗-美坦新偶联物、曲妥珠单抗、紫杉醇4种药物和空白对照磷酸盐缓冲液,考察药物的抑瘤效果。结果:流式细胞仪检测结果显示,SK-OV-3、NCI-N87、SK-BR-3、Calu-3、HCC1954、BT-474这6株肿瘤细胞的HER2表达量较高,每个细胞表面HER2受体数在43~80万个,比另外3株MDA-MB-231、MCF-7、MDA-MB-468肿瘤细胞的HER2表达水平高50倍以上。药物对9种肿瘤细胞的增殖抑制活性结果显示,抗HER2-ADC对HER2高表达的细胞有很强的抑制细胞生长活性,对SK-OV-3、NCI-N87、SK-BR-3、Calu-3、HCC1954、BT-474的半数抑制浓度分别为46、17、17、161、125、50 pmol/L。在动物体内药效试验中,抗HER2-ADC在所有HER2阳性表达的肿瘤模型中都表现了较强的呈剂量依赖的抗肿瘤活性,在NCI-N87异种移植肿瘤模型中,与曲妥珠单抗及曲妥珠单抗-美坦新偶联物相比,相同剂量的抗HER2-ADC表现出更好的抗肿瘤活性,其相对肿瘤增殖率约为二者的1/30至1/20,在HCC1954模型中表现出肿瘤的完全消退和治愈效果;抗HER2-ADC对HER2低表达MDA-MB-468肿瘤细胞移植瘤模型没有效果。与曲妥珠单抗-美坦新偶联物相比,抗HER2-ADC表现出相同或更好的抗肿瘤活性。结论:用非天然氨基酸定点偶联技术获得的抗HER2-ADC在细胞体外和动物体内实验中对HER2高表达的肿瘤具有明显的抑制效果。
中图分类号:
- R966
| [1] | Peters C, Brown S . Antibody-drug conjugates as novel anti-cancer chemotherapeutics[J]. Biosci Rep, 2015,35(4):1042-1061. |
| [2] | Bouchard H, Viskov C, Garcia-Echeverria C . Antibody-drug conjugates: a new wave of cancer drugs[J]. Bioorg Med Chemi Lett, 2014,24(23):5357-5363. |
| [3] | Hamblett KJ, Senter PD, Chace DF , et al. Effects of drug loading on the antitumor activity of a monoclonal antibody drug conjugate[J]. Clin Cancer Res, 2004,10(20):7063-7070. |
| [4] | Wang L, Amphlett G, Blättler WA , et al. Structural characterization of the maytansinoid-monoclonal antibody immunoconjugate, huN901-DM1, by mass spectrometry[J]. Protein Sci, 2005,14(9):2436-2446. |
| [5] | Sun MM, Beam KS, Cerveny CG , et al. Reduction-alkylation strategies for the modification of specific monoclonal antibody disulfides[J]. Bioconjug Chem, 2005,16(5):1282-1290. |
| [6] | Junutula JR, Raab H, Clark S , et al. Site-specific conjugation of a cytotoxic drug to an antibody improves the therapeutic index[J]. Nat Biotechnol, 2008,26(8):925-932. |
| [7] | Tian F, Lu Y, Manibusan A , et al. A general approach to site-specific antibody drug conjugates[J]. Proc Natl Acad Sci USA, 2014,111(5):1766-1771. |
| [8] | Li X, Yang J, Rader C . Antibody conjugation via one and two C-terminal selenocysteines[J]. Methods, 2013,65(1):133-138. |
| [9] | Tang F, Yang Y, Tang Y , et al. One-pot N-glycosylation remodeling of IgG with non-natural sialylglycopeptides enables glycosite-specific and dual-payload antibody-drug conjugates[J]. Org Biomol Chem, 2016,14(40):9501-9518. |
| [10] | Dennler P, Fischer E, Schibli R . Antibody conjugates: from heterogeneous populations to defined reagents[J]. Antibodies, 2015,4(3):197-224. |
| [11] | Shen BQ, Xu K, Liu L , et al. Conjugation site modulates the in vivo stability and therapeutic activity of antibody-drug conjugates[J]. Nat Biotechnol, 2012,30(2):184-189. |
| [12] | Wang L, Brock A, Schultz PG , et al. Expanding the genetic code of Escherichia coli[J]. Science, 2001,292(5516):498-500. |
| [13] | Slamon DJ, Leyland-Jones B, Shak B , et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2[J]. N Engl J Med, 2001,344(11):783-792. |
| [14] | Meden H, Kuhn W . Overexpression of the oncogene c-erbB-2 (HER2/neu) in ovarian cancer: a new prognostic factor[J]. Eur J Obstet Gynecol Reprod Biol, 1997,71(2):173-179. |
| [15] | Al-toub M, Vishnubalaji R, Hamam R , et al. CDH1 and IL1-beta expression dictates FAK and MAPKK-dependent cross-talk between cancer cells and human mesenchymal stem cells[J]. Stem Cell Res Ther, 2015,6(1):135. |
| [16] | Yang J, Yang G, Hou G , et al. Scutellaria barbata D. Don polysaccharides inhibit the growth of Calu-3 xenograft tumors via suppression of the HER2 pathway and angiogenesis[J]. Oncol Lett, 2015,9(6):2721-2725. |
| [17] | Nonagase Y, Yonesaka K, Kawakami H , et al. Heregulin-expressing HER2-positive breast and gastric cancer exhibited heterogeneous susceptibility to the anti-HER2 agents lapatinib, trastuzumab and T-DM1[J]. Oncotarget, 2016,7(51):84860-84871. |
| [18] | de Vlieghere E, Carlier C, Ceelen W , et al. Data on in vivo selection of SK-OV-3 luc ovarian cancer cells and intraperitoneal tumor formation with low inoculation numbers[J]. Data Brief, 2016,6:542-549. |
| [19] | Atnip AA, Sigurdson GT, Bomser J , et al. Time, concentration, and pH-dependent transport and uptake of anthocyanins in a human gastric epithelial (NCI-N87) cell line[J]. Int J Mol Sci, 2017,18(2):446. |
| [20] | Trail PA, Dubowchik GM, Lowinger TB . Antibody drug conjugates for treatment of breast cancer: novel targets and diverse approaches in ADC design[J]. Pharmacol Ther, 2018,181:126-142. |
| [21] | Behrens CR, Liu B . Methods for site-specific drug conjugation to antibodies[J]. MAbs, 2014,6(1):46-53. |
| [22] | Jain N, Smith SW, Ghone S , et al. Current ADC linker chemistry[J]. Pharm Res, 2015,32(11):3526-3540. |
| [23] | Diamantis N, Banerji U . Antibody-drug conjugates: an emerging class of cancer treatment[J]. Br J Cancer, 2016,114(4):362-367. |
| [24] | Panowski S, Bhakta S, Raab H , et al. Site-specific antibody drug conjugates for cancer therapy[J]. MAbs, 2014,6(1):34-45. |
| [25] | Fishkin N, Maloney EK, Chari RV , et al. A novel pathway for maytansinoid release from thioether linked antibody-drug conjugates (ADCs) under oxidative conditions[J]. Chem Commun, 2011,47(38):10752-10754. |
| [26] | Ponte JF, Sun X, Yoder NC , et al. Understanding how the stabi-lity of the thiol-maleimide linkage impacts the pharmacokinetics of lysine-linked antibody-maytansinoid conjugates[J]. Bioconjug Chem, 2016,27(7):1588-1598. |
| No related articles found! |
|
||