北京大学学报(医学版) ›› 2019, Vol. 51 ›› Issue (5): 893-899. doi: 10.19723/j.issn.1671-167X.2019.05.017
胞内转运对根尖牙乳头干细胞表面CXC趋化因子受体4表达的影响
- 北京大学口腔医学院·口腔医院,牙体牙髓科 国家口腔疾病临床医学研究中心 口腔数字化医疗技术和材料国家工程实验室 口腔数字医学北京市重点实验室,北京 100081
Role of endocytosis in cell surface CXC chemokine receptor 4 expression of stem cells from apical papilla
Xin-yun YAO,Xiao-min GAO,Xiao-ying ZOU,Lin YUE( )
)
- Department of Cariology and Endodontology, Peking University School and Hospital of Stomatology & National Clinical Research Center for Oral Diseases & National Engineering Laboratory for Digital and Material Technology of Stomatology & Beijing Key Laboratory of Digital Stomatology, Beijing 100081, China
摘要:
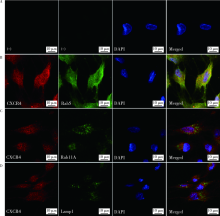
目的:探讨胞内转运途径受到抑制后,根尖牙乳头干细胞(stem cells from apical papilla, SCAP)表面CXC趋化因子受体4(CXC chemokine receptor 4, CXCR4)表达的变化,为了解SCAP迁移机制提供实验依据。方法:采用免疫荧光共染色方法和原位邻近连接技术(proximity ligation assay, PLA)检测SCAP胞内CXCR4与细胞胞内转运相关细胞器标记蛋白的共定位,检测指标包括早期胞内体标记蛋白Rab5、循环胞内体标记蛋白Rab11A、溶酶体标记蛋白Lamp1。分别采用80 μmol/L的内吞抑制剂Blebbistatin、80 μmol/L的内吞抑制剂Dyanasore对SCAP进行预处理1 h,流式细胞分析方法检测表面阳性表达CXCR4的SCAP所占百分比,使用单因素方差分析进行统计学分析。结果:免疫荧光共染色结果显示,CXCR4在SCAP胞内的表达位置与早期胞内体标记物Rab5、循环胞内体标记物Rab11A的表达位置重合,小部分与溶酶体标记物Lamp1的表达位置重合。PLA结果显示,CXCR4在SCAP胞内与Rab5、Rab11A、Lamp1均存在共定位。阴性对照组SCAP CXCR4阳性表达的细胞为 0.13%±0.10%, 经Blebbistatin、Dynasore抑制内吞后,表面CXCR4阳性表达的SCAP显著增多,分别为13.34%±1.31%、4.03%±0.92%,差异有统计学意义(F=161.762,P<0.001), 且Blebbistatin组多于Dynasore组,差异有统计学意义(P<0.001)。结论:采用内吞抑制剂抑制CXCR4的胞内转运途径,可使表面表达CXCR4的SCAP增多,提升了SCAP迁移的潜能。
中图分类号:
- R78
| [1] | Sonoyama W, Liu Y, Yamaza T , et al. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study[J]. J Endod, 2008,34(2):166-171. |
| [2] | Sonoyama W, Liu Y, Fang D , et al. Mesenchymal stem cell-mediated functional tooth regeneration in swine[J]. PLoS One, 2006,1(1):e79. |
| [3] | Liu JY, Chen X, Yue L , et al. CXC Chemokine receptor 4 is expressed paravascularly in apical papilla and coordinates with stromal cell-derived factor-1alpha during transmigration of stem cells from apical papilla[J]. J Endod, 2015,41(9):1430-1436. |
| [4] | Yang C, Li X, Sun L , et al. Potential of human dental stem cells in repairing the complete transection of rat spinal cord[J]. J Neural Eng, 2017,14(2):26005. |
| [5] | Marquez-Curtis LA, Janowska-Wieczorek A . Enhancing the migration ability of mesenchymal stromal cells by targeting the SDF-1/CXCR4 axis [J/OL]. Biomed Res Int, 2013, 2013: 561098[ 2018- 05- 01]. . |
| [6] | Wynn RF, Hart CA, Corradi-Perini C , et al. A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow[J]. Blood, 2004,104(9):2643-2645. |
| [7] | Jiang L, Peng WW, Li LF , et al. Isolation and identification of CXCR4-positive cells from human dental pulp cells[J]. J Endod, 2012,38(6):791-795. |
| [8] | Zhang Y, Foudi A, Geay JF , et al. Intracellular localization and constitutive endocytosis of CXCR4 in human CD34+ hemato-poietic progenitor cells[J]. Stem Cells, 2004,22(6):1015-1029. |
| [9] | Hutagalung AH, Novick PJ . Role of rab gtpases in membrane traffic and cell physiology[J]. Physiol Rev, 2011,91(1):119-149. |
| [10] | Voss S, Li F, Rätz A , et al. Spatial cycling of rab GTPase, driven by the GTPase cycle, controls rab’s subcellular distribution[J]. Biochemistry, 2019,58(4):276-285. |
| [11] | Pelekanos RA, Ting MJ, Sardesai VS , et al. Intracellular trafficking and endocytosis of CXCR4 in fetal mesenchymal stem/stroal cells[J/OL]. BMC Cell Biol, 2014, 15: 15[2018-05-01]. |
| [12] | Huang GT, Gronthos S, Shi S . Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine[J]. J Dent Res, 2009,88(9):792-806. |
| [13] | 刘敬一, 邹晓英, 陈雪 , 等. 脂多糖对人根尖牙乳头干细胞中基质细胞衍生因子1表达的影响[J]. 中华口腔医学杂志, 2015,50(6):346-351. |
| [14] | Stenmark H . Rab GTPases as coordinators of vesicle traffic[J]. Nat Rev Mol Cell Bio, 2009,10(8):513-525. |
| [15] | Sönnichsen B, De Renzis S, Nielsen E , et al. Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11[J]. J Cell Bio, 2000,149(4):901-914. |
| [16] | Rajapakshe AR, Podyma-Inoue KA, Terasawa K , et al. Lysosome-associated membrane proteins (LAMPs) regulate intracellular positioning of mitochondria in MC3T3-E1 cells[J]. Exp Cell Res, 2015,331(1):211-222. |
| [17] | Marchese A, Chen C, Kim YM , et al. The ins and outs of G protein-coupled receptor trafficking[J]. Trends Biochem Sci, 2003,28(7):369-376. |
| [18] | Signoret N, Oldridge J, Pelchen-Matthews A , et al. Phorbol esters and SDF-1 induce rapid endocytosis and down modulation of the chemokine receptor CXCR4[J]. J Cell Biol, 1997,139(3):651-664. |
| [19] | Chandrasekar I, Goeckeler ZM, Turney SG , et al. Nonmuscle myosin Ⅱ is a critical regulator of clathrin-mediated endocytosis[J]. Traffic, 2014,15(4):418-432. |
| [20] | Morlot S, Roux A . Mechanics of dynamin-mediated membrane fission[J]. Annu Rev Biophys, 2013,42:629-649. |
| [21] | Linares-Clemente P, Rozas JL, Mircheski J , et al. Different dynamin blockers interfere with distinct phases of synaptic endocytosis during stimulation in motoneurones[J]. J Physiol, 2015,593(13):2867-2888. |
| [22] | Cepeda EB, Dediulia T, Fernando J , et al. Mechanisms regulating cell membrane localization of the chemokine receptor CXCR4 in human hepatocarcinoma cells[J]. Biochim Biophys Acta, 2015,1853(5):1205-1218. |
| [23] | Jiang J, Kolpak AL, Bao ZZ . Myosin IIB isoform plays an essential role in the formation of two distinct types of macropinosomes[J]. Cytoskeleton (Hoboken), 2010,67(1):32-42. |
| [24] | Sandvig K, Kavaliauskiene S, Skotland T . Clathrin-independent endocytosis: an increasing degree of complexity[J]. Histochem Cell Biol, 2018,150(2):107-118. |
| [1] | 张瑶,郭金鑫,战世佳,洪恩宇,杨慧,贾安娜,常艳,郭永丽,张璇. 富含半胱氨酸和甘氨酸蛋白2在神经母细胞瘤恶性进展中的功能和机制[J]. 北京大学学报(医学版), 2024, 56(3): 495-504. |
| [2] | 曹钟,岑红兵,赵建红,梅俊,秦灵芝,廖伟,敖启林. 胰腺神经内分泌肿瘤和实性假乳头状肿瘤中INSM1和SOX11的表达及意义[J]. 北京大学学报(医学版), 2023, 55(4): 575-581. |
| [3] | 王磊,韩天栋,江卫星,李钧,张道新,田野. 主动迁移技术与原位碎石技术在输尿管软镜治疗1~2 cm输尿管上段结石中的安全性和有效性比较[J]. 北京大学学报(医学版), 2023, 55(3): 553-557. |
| [4] | 高晓敏,邹晓英,岳林. 根尖牙乳头干细胞摄取外泌体的介导途径[J]. 北京大学学报(医学版), 2020, 52(1): 43-50. |
| [5] | 张帆,燕太强,郭卫. Rasfonin抑制骨肉瘤细胞143B的增殖和迁移[J]. 北京大学学报(医学版), 2019, 51(2): 234-238. |
| [6] | 胡风战,原婉琼,王晓林,秦彩朋,盛正祚,杜依青,殷华奇,徐涛. 敲减CMTM3增强前列腺癌细胞系PC3迁移与侵袭能力[J]. 北京大学学报(医学版), 2016, 48(4): 594-597. |
| [7] | 杨杰, 赵玉鸣, 王文君, 贾维茜, 葛立宏. 犬根尖牙乳头干细胞的分离培养和生物学特性[J]. 北京大学学报(医学版), 2012, 44(6): 921-926. |
| [8] | 陈鑫磊, 边曦, 秦泽莲. Periostin在酸性环境下对人脐静脉内皮细胞功能的影响[J]. 北京大学学报(医学版), 2011, 43(6): 855-860. |
|
||