北京大学学报(医学版) ›› 2019, Vol. 51 ›› Issue (5): 900-906. doi: 10.19723/j.issn.1671-167X.2019.05.018
3种口腔颌面部来源的间充质干细胞成血管内皮分化潜能的比较研究
谢静1,赵玉鸣1,饶南荃1,汪晓彤2,方滕姣子1,李晓霞1,翟越1,李静芝1,葛立宏1,王媛媛1,△( )
)
- 1. 北京大学口腔医学院·口腔医院,儿童口腔科 国家口腔疾病临床医学研究中心 口腔数字化医疗技术和材料国家工程实验室 口腔数字医学北京市重点实验室,北京 100081
2. 北京大学口腔医学院·口腔医院,急诊科 国家口腔疾病临床医学研究中心 口腔数字化医疗技术和材料国家工程实验室 口腔数字医学北京市重点实验室,北京 100081
Comparative study of differentiation potential of mesenchymal stem cells derived from orofacial system into vascular endothelial cells
Jing XIE1,Yu-ming ZHAO1,Nan-quan RAO1,Xiao-tong WANG2,Teng-jiao-zi FANG1,Xiao-xia LI1,Yue ZHAI1,Jing-zhi LI1,Li-hong GE1,Yuan-yuan WANG1,△( )
)
- 1. Department of Pediatric Dentistry, Peking University School and Hospital of Stomatology & National Clinical Research Center for Oral Diseases & National Engineering Laboratory for Digital and Material Technology of Stomatology & Beijing Key Laboratory of Digital Stomatology, Beijing 100081, China
2. Department of Oral Emergency, Peking University School and Hospital of Stomatology & National Clinical Research Center for Oral Diseases & National Engineering Laboratory for Digital and Material Technology of Stomatology & Beijing Key Laboratory of Digital Stomatology, Beijing 100081, China
摘要:
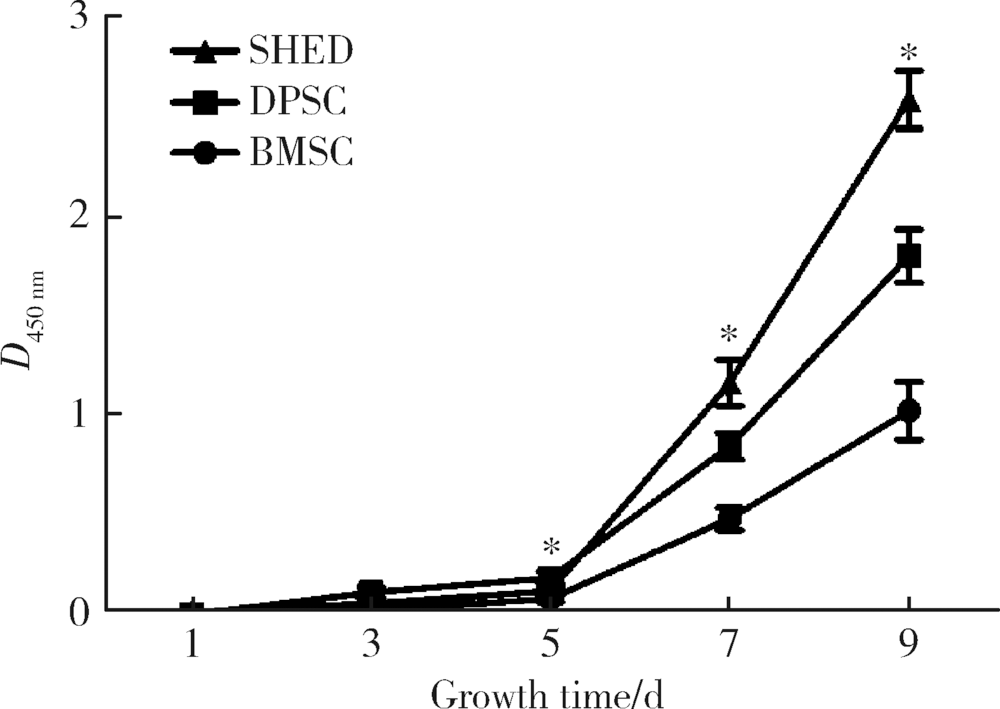
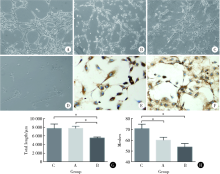
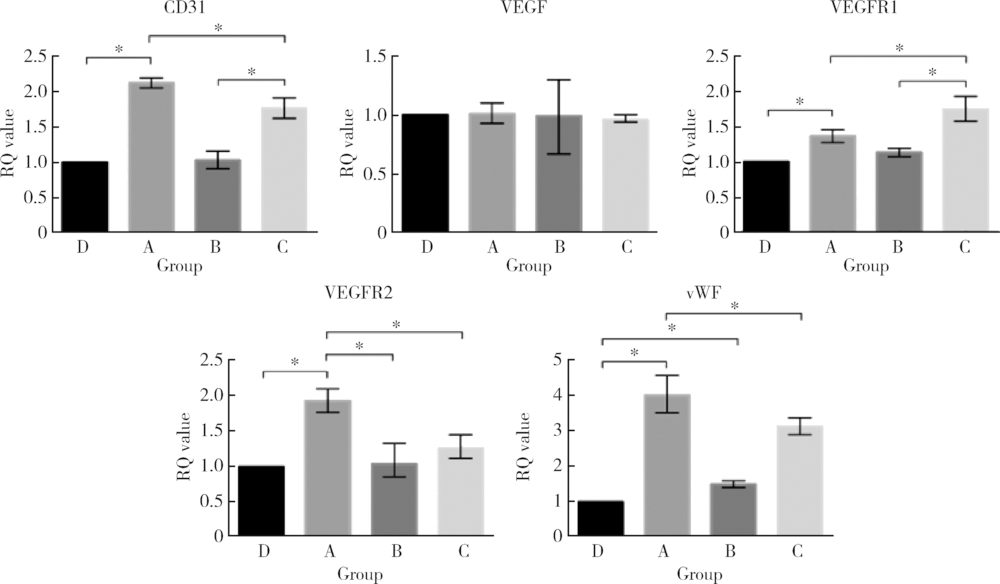
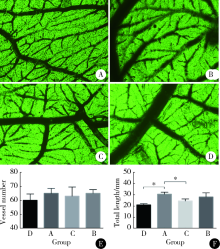
目的:研究来自口腔颌面部的脱落乳牙牙髓干细胞(stem cells from human exfoliated deciduous teeth, SHED)、牙髓干细胞(dental pulp stem cells, DPSC)和颌骨骨髓间充质干细胞(bone marrow mesenchymal stem cell, BMSC)的增殖能力及成血管内皮细胞分化潜能,为血管组织工程再生种子细胞的选择提供依据。方法:取临床乳牙和恒牙牙髓组织及颌骨组织并采用酶消化法分离培养相应的间充质干细胞,流式细胞技术检测间充质干细胞相关表面抗原的表达。采用CCK-8 (cell counting kit-8)法检测细胞的增殖能力。通过Matrigel三维培养技术诱导间充质干细胞成血管内皮细胞分化,并通过管腔计数及real-time PCR技术比较3种间充质干细胞的成血管内皮细胞分化能力。采用鸡胚绒毛尿囊膜(chick embryo chorioallantoic membrane, CAM)技术观察3种不同间充质干细胞新生血管能力。结果:3种干细胞均阳性表达CD73、CD90、CD105、CD146,阴性表达 CD34、CD45,符合间充质干细胞表面标记物的表达规律。SHED和DPSC的CD146表达率多于BMSC,CCK-8法检测显示SHED的增殖能力最强。诱导后的3种细胞均可在Matrigel基质胶上形成管腔样结构,诱导后SHED和BMSC形成的血管总长度大于DPSC,SHED形成的管腔数多于BMSC和DPSC。real-time PCR 结果显示几种成血管相关的细胞因子在不同细胞间表达存在差别。诱导后SHED的CD31、VEGFR2、vWF表达显著高于另外两种细胞。BMSC的VEGFR1表达量高于其他组,SHED高于DPSC。VEGF的表达在4组之间差异无统计学意义。3种细胞在CAM上计数新生血管数目显示较空白对照组差异无统计学意义,SHED组血管总长度较空白对照和BMSC大。结论:SHED、DPSC及 BMSC均能向成血管内皮细胞方向诱导分化且在Matrigel培养基上形成血管和管腔,SHED具有更强的分化和形成管腔的能力,同时SHED较BMSC及DPSC具有更强的增殖能力。
中图分类号:
- R78
| [1] | Sakaguchi Y, Sekiya I, Yagishita K , et al. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source[J]. Arthritis Rheumatol, 2005,52(8):2521. |
| [2] | Folkman J . Angiogenesis in cancer, vascular, rheumatoid and other disease[J]. Nat Med, 1995,1(1):27. |
| [3] | Carmeliet P . Angiogenesis in life, disease and medicine[J]. Nature, 2005,438(7070):932. |
| [4] | Canan A, Huseyin A, Riza KA , et al. Angiogenesis in inflammatory bowel disease[J]. In J Inflammation, 2015,2015(3):970890. |
| [5] | Tateishi-Yuyama E, Matsubara H, Murohara T . Therapeutic angiogenesis for patients with limb ischemia by autologous transplantation of bone-marrow cells: a pilot study and a randomized controlled trial[J]. Acc Current J Rev, 2002,360(9331):427. |
| [6] | Hou L, Kim JJ, Woo YJ , et al. Stem cell-based therapies to promote angiogenesis in ischemic cardiovascular disease[J]. Am J Physiol, 2016,310(4):H455. |
| [7] | Shi S, Gronthos S . Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp[J]. J Bone Miner Res, 2003,18(4):696-704. |
| [8] | Belotti A, Elli E, Speranza T , et al. Circulating endothelial cells and endothelial activation in essential thrombocythemia: results from CD146 + immunomagnetic enrichment: flow cytometry and soluble E-selectin detection [J]. Am J Hematol, 2012,87(3):319. |
| [9] | Jouve N, Despoix N, Espeli M , et al. The involvement of CD146 and its novel ligand galectin-1 in apoptotic regulation of endothelial cells[J]. J Biol Chem, 2013,288(4):2571-2579. |
| [10] | Nakamura S, Yamada Y, Katagiri W , et al. Stem cell proliferation pathways comparison between human exfoliated deciduous teeth and dental pulp stem cells by gene expression profile from promising dental pulp[J]. J Endodont, 2009,35(11):1536-1542. |
| [11] | Duttenhoefer F, Lara d FR, Meury T, et al. 3D scaffolds co-seeded with human endothelial progenitor and mesenchymal stem cells: evidence of prevascularisation within 7 days[J]. Eur Cells Mater, 2013,26(4):49. |
| [12] | Moore MC, Pandolfi V, Mcfetridge PS . Novel human-derived extracellular matrix induces in vitro, and in vivo, vascularization and inhibits fibrosis[J]. Biomaterials, 2015,49:37. |
| [13] | Nourse MB, Halpin DE, Scatena M , et al. VEGF induces differentiation of functional endothelium from human embryonic stem cells: implications for tissue engineering[J]. Arterioscler Thromb Vas Biol, 2010,30(1):80-89. |
| [14] | Oswald J, Boxberger S, Jørgensen B , et al. Mesenchymal stem cells can be differentiated into endothelial cells in vitro[J]. Stem Cells, 2004,22(3):377. |
| [15] | Sugiyama M, Iohara K, Wakita H , et al. Dental pulp-derived CD31 -/CD146 - side population stem/progenitor cells enhance recovery of focal cerebral ischemia in rats [J]. Tissue Eng Part A, 2011,17(9/10):1303-1311. |
| [16] | Park S, Sorenson CM, Sheibani N . PECAM-1 isoforms, eNOS and endoglin axis in regulation of angiogenesis[J]. Clin Sci, 2015,129(3):217. |
| [17] | Zhang Z, Neiva KG, Lingen MW , et al. VEGF-dependent tumor angiogenesis requires inverse and reciprocal regulation of VEGFR1 and VEGFR2[J]. Cell Death Differ, 2010,17(3):499. |
| [18] | Ruszkowskaciastek B, Sokup A, Socha M W , et al. A preliminary evaluation of VEGF-A, VEGFR1 and VEGFR2 in patients with well-controlled type 2 diabetes mellitus[J]. J Zhejiang Univ Sci B, 2014,15(6):575-581. |
| [19] | Rondaij MG, Bierings R, Kragt A , et al. Dynamics and plasticity of Weibel-Palade bodies in endothelial cells[J]. Arterioscler Thromb Vasc Biol, 2006,26(5):1002. |
| [20] | Kleibeuker EA, Schulkens IA, Castricum KC , et al. Examination of the role of galectins during in vivo angiogenesis using the chick chorioallantoic membrane assay[J]. Methods Mol Biol, 2015,1207:305. |
| [21] | Dehelean CA, Feflea S, Gheorgheosu D , et al. Anti-angiogenic and anti-cancer evaluation of betulin nanoemulsion in chicken chorioallantoic membrane and skin carcinoma in BALB/c mice[J]. J Biomed Nanotechnol, 2013,9(4):577-589. |
| [1] | 张瑶,郭金鑫,战世佳,洪恩宇,杨慧,贾安娜,常艳,郭永丽,张璇. 富含半胱氨酸和甘氨酸蛋白2在神经母细胞瘤恶性进展中的功能和机制[J]. 北京大学学报(医学版), 2024, 56(3): 495-504. |
| [2] | 叶雨阳,岳林,邹晓英,王晓燕. 成牙本质方向分化牙髓干细胞外泌体形态及微小RNA表达谱特征[J]. 北京大学学报(医学版), 2023, 55(4): 689-696. |
| [3] | 刘媛,原婉琼,李婷,王平章,吕平,吴利新,阮国瑞,韩文玲,莫晓宁. 敲减CMTM3增加急性B淋巴细胞白血病细胞对伊马替尼敏感性[J]. 北京大学学报(医学版), 2022, 54(6): 1238-1243. |
| [4] | 高晓敏,邹晓英,岳林. 根尖牙乳头干细胞摄取外泌体的介导途径[J]. 北京大学学报(医学版), 2020, 52(1): 43-50. |
| [5] | 王子成,程立,吕同德,苏黎,林健,周利群. 炎症因子预处理的脂肪干细胞可明显抑制外周血单个核细胞增殖[J]. 北京大学学报(医学版), 2018, 50(4): 590-594. |
| [6] | 唐旭,赵卫红,宋琴琴,殷华奇,杜依青,盛正祚,王强,张晓威,李清,刘士军,徐涛. SOX10对前列腺癌细胞增殖及侵袭的影响[J]. 北京大学学报(医学版), 2018, 50(4): 602-606. |
| [7] | 汪晓彤,饶南荃,方腾姣子,赵玉鸣,葛立宏. 乳牙牙髓干细胞CD146阳性/阴性细胞亚群生物学特性的比较[J]. 北京大学学报(医学版), 2018, 50(2): 284-292. |
| [8] | 陈玮, 胡凡磊, 刘洪江, 徐丽玲, 李英妮, 栗占国. 类风湿关节炎患者髓系来源的抑制细胞促进自身B细胞增殖[J]. 北京大学学报(医学版), 2017, 49(5): 819-823. |
| [9] | 蔡燚,郭浩,李汉忠,王文达,张玉石. 结节性硬化症细胞株TSC2-/- MEFs和正常细胞株TSC2+/+ MEFs微小RNA表达谱的差异分析[J]. 北京大学学报(医学版), 2017, 49(4): 580-584. |
| [10] | 贾维茜,赵玉鸣,葛立宏. 人重组转化生长因子β1促进牙髓干细胞的增殖和矿化[J]. 北京大学学报(医学版), 2017, 49(4): 680-681. |
| [11] | 杨迪,许珺辉,邓芙蓉,郭新彪. 纳米银对皮肤细胞半通道活性的影响及其在细胞增殖抑制中的作用[J]. 北京大学学报(医学版), 2017, 49(3): 371-375. |
| [12] | 高翔,陈香梅,张婷,张静,陈茉,郭正阳,石岩岩,鲁凤民,丁士刚. 巨噬细胞加帽蛋白与胃癌细胞增殖及迁移能力的关系[J]. 北京大学学报(医学版), 2017, 49(3): 489-494. |
| [13] | 司马梓涵,洪瑛瑛,李铁军. PTCH1基因突变对牙源性角化囊性瘤上皮细胞增殖的影响[J]. 北京大学学报(医学版), 2017, 49(3): 522-526. |
| [14] | 隋华欣, 吕培军, 王宇光, 王勇, 孙玉春. 低能量激光照射对人脂肪基质细胞增殖分化的影响[J]. 北京大学学报(医学版), 2017, 49(2): 337-343. |
| [15] | 李静文,殷晓晖,栾庆先. 不同代次诱导多能干细胞增殖及牙周定向分化的能力[J]. 北京大学学报(医学版), 2017, 49(1): 16-024. |
|
||