北京大学学报(医学版) ›› 2020, Vol. 52 ›› Issue (3): 438-443. doi: 10.19723/j.issn.1671-167X.2020.03.007
苯并[a]芘对脑内多巴胺能神经元和α-突触核蛋白的影响及其机制
- 北京大学公共卫生学院劳动卫生与环境卫生学系,北京 100191
Effect of benzo(a)pyrene on dopaminergic neurons and α-synuclein in brain and its mechanism involved
Yu-ze QI,Hui-hui QUAN,Wei-xing XU,Qing-ru LI,Hui ZHOU( )
)
- Department of Occupational and Environmental Health, Peking University School of Public Health, Beijing 100191, China
摘要:
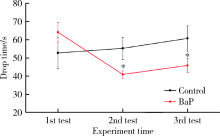
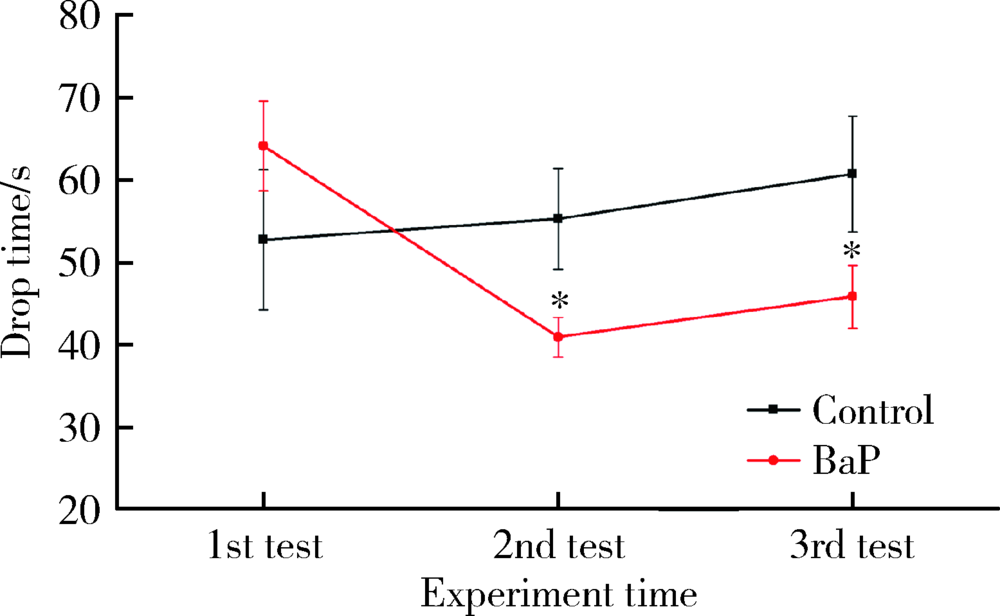
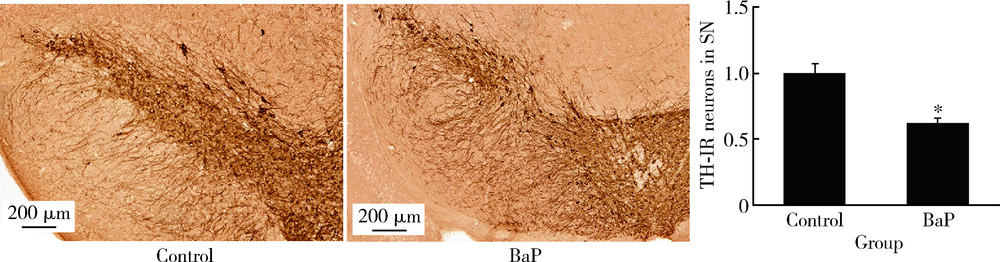
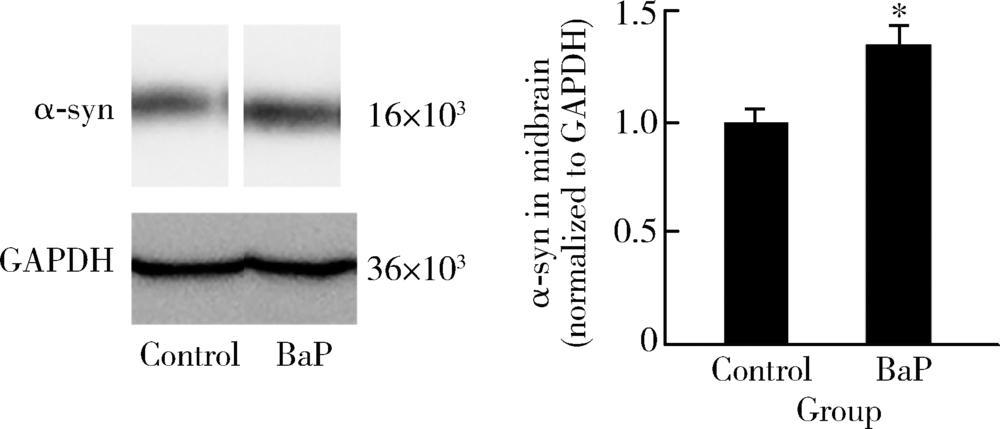
目的 分析苯并[a]芘[benzo(a)pyrene,BaP]暴露对帕金森病病理特征多巴胺能神经元和α-突触核蛋白表达的影响,并探讨可能的机制。方法 8月龄人源SNCA转基因小鼠随机分为BaP染毒组和对照组,分别腹腔注射1.0 mg/kg体质量的BaP和玉米油溶剂,连续注射60 d。通过转轮实验观察小鼠运动功能障碍状况,通过免疫组织化学与免疫蛋白印迹实验观察BaP对多巴胺能神经元和α-突触核蛋白的影响,采用实时荧光定量PCR法检测相关mRNA的表达。研究中涉及的基因主要与神经递质转运蛋白、神经递质受体、细胞自噬和α-突触核蛋白聚集与降解相关。结果 BaP染毒后,转轮实验中小鼠运动时间显著降低(P<0.05),小鼠黑质多巴胺能神经元明显减少,为对照组的62%(P<0.05),中脑α-突触核蛋白表达增多,为对照组的1.36倍(P<0.05)。BaP染毒后,小鼠中脑14种mRNA表达显著下调(P均<0.05),主要涉及α-突触核蛋白降解与细胞自噬、神经元转运体、神经递质受体等功能;而Synphilin-1表达显著上调(P<0.01),与α-突触核蛋白合成有关。结论 BaP暴露抑制神经递质受体、多巴胺转运体蛋白功能和细胞自噬作用,阻碍α-突触核蛋白降解,从而诱导黑质多巴胺能神经元变性死亡和α-syn聚集体形成,增加帕金森病发病风险。
中图分类号:
- R12
| [1] |
Saunders C, Shockley D, Knuckles M. Behavioral effects induced by acute exposure to benzo(a)pyrene in F-344 rats[J]. Neurotox Res, 2001,3(6):557-579.
pmid: 15111245 |
| [2] |
Qiu CY, Cheng SQ, Xia YY, et al. Effects of subchronic benzo(a)pyrene exposure on neurotransmitter receptor gene expression in the rat hippocampus related with spatial learning and memory change[J]. Toxicology, 2011,289(2-3):83-90.
doi: 10.1016/j.tox.2011.07.012 pmid: 21839799 |
| [3] | Gao DX, Wu MF, Wang CG, et al. Chronic exposure to low benzo[a]pyrene level causes neurodegenerative disease-like syndromes in zebrafish (Danio rerio)[J]. Aquat Toxicol, 2015,167(10):200-208. |
| [4] |
Burre J, Sharma M, Tsetsenis T, et al. Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro[J]. Science, 2010,329(5999):1663-1667.
doi: 10.1126/science.1195227 pmid: 20798282 |
| [5] | Naughton C, O’toole D, Kirik D, et al. Interaction between subclinical doses of the Parkinson’s disease associated gene, alpha-synuclein, and the pesticide, rotenone, precipitates motor dysfunction and nigrostriatal neurodegeneration in rats[J]. Behav Brain Res, 2017,316(1):160-168. |
| [6] | Wilson W, Shapiro L, Bradner J, et al. Developmental exposure to the organochlorine insecticide endosulfan damages the nigrostriatal dopamine system in male offspring[J]. Neurotoxicology, 2014,44(9):279-287. |
| [7] |
Ali S, Rajini P. Elicitation of dopaminergic features of Parkinson’s disease in C. elegans by monocrotophos, an organophosphorous insecticide[J]. CNS Neurol Disord Drug Targets, 2012,11(8):993-1000.
doi: 10.2174/1871527311211080008 pmid: 23244418 |
| [8] | Palacios N, Fitzgerald K, Hart J, et al. Air pollution and risk of Parkinson's disease in a large prospective study of men[J]. Environ Health Perspect, 2017,125(8):1-7. |
| [9] | Lee P, Liu L, Sun Y, et al. Traffic-related air pollution increased the risk of Parkinson’s disease in Taiwan: A nationwide study[J]. Environ Int, 2016,96(11):75-81. |
| [10] |
Das M, Seth P, Mukhtar H. Distribution of benzo(a)pyrene in discrete regions of rat brain[J]. Bull Environ Contam Toxicol, 1985,35(4):500-504.
pmid: 4052652 |
| [11] | Michaelson J, Trump S, Rudzok S, et al. Transcriptional signatures of regulatory and toxic responses to benzo-[a]-pyrene exposure[J]. BMC Genomics, 2011,12(10):502-515. |
| [12] | Jayasekara S, Sharma R, Drown D. Effects of benzo[a]pyrene on steady-state levels of biogenic amines and metabolizing enzymes in mouse brain regions[J]. Ecotoxicol Environ Saf, 1992,24(1):1-12. |
| [13] | Naoi M, Maruyama W, Nagy G. Dopamine-derived salsolinol derivatives as endogenous monoamine oxidase inhibitors: occurrence, metabolism and function in human brains[J]. Neurotoxicology, 2004,25(1-2):193-204. |
| [14] | Stephanou P, Konstandi M, Pappas P, et al. Alterations in central monoaminergic neurotransmission induced by polycyclic aromatic hydrocarbons in rats[J]. Eur J Drug Metab Pharmacokinet, 1998,23(4):475-481. |
| [15] | Bouayed J, Desor F, Rammal H, et al. Effects of lactational exposure to benzo[alpha]pyrene (B[alpha]P) on postnatal neurodevelopment, neuronal receptor gene expression and behaviour in mice[J]. Toxicology, 2009,259(3):97-106. |
| [16] |
Guillot T, Richardson J, Wang M, et al. PACAP38 increases vesicular monoamine transporter 2 (VMAT2) expression and attenuates methamphetamine toxicity[J]. Neuropeptides, 2008,42(4):423-434.
doi: 10.1016/j.npep.2008.04.003 |
| [17] | Giasson B, Duda J, Quinn S, et al. Neuronal alpha-synucleinopathy with severe movement disorder in mice expressing A53T human alpha-synuclein[J]. Neuron, 2002,34(4):521-533. |
| [18] | Webb J, Ravikumar B, Atkins J, et al. Alpha-synuclein is degraded by both autophagy and the proteasome[J]. J Biol Chem, 2003,278(27):25009-25013. |
| [19] | Beilina A, Cookson M. Genes associated with Parkinson’s disease: Regulation of autophagy and beyond[J]. J Neurochem, 2016,139(10):91-107. |
| [20] | Crews L, Spencer B, Desplats P, et al. Selective molecular alterations in the autophagy pathway in patients with Lewy body disease and in models of alpha-synucleinopathy[J]. PLoS One, 2010,5(2):e9313. |
| [1] | 权会会,徐卫星,祁宇泽,李清如,周辉,黄婧. 模拟肽Gap27抑制缝隙连接蛋白43在帕金森病小鼠模型中的作用[J]. 北京大学学报(医学版), 2022, 54(3): 421-426. |
| [2] | 刘小璇,张朔,刘娜,孙阿萍,张英爽,樊东升. 震颤分析用于早期帕金森病的诊断价值[J]. 北京大学学报(医学版), 2019, 51(6): 1096-1102. |
| [3] | 韩济生. 疼痛、药物成瘾和神经退行性疾病最新研究进展[J]. 北京大学学报(医学版), 2009, 41(3): 249-254. |
| [4] | 高红, 王建军, 张蔚, 蒋玉辉, 牛东滨, 王晓民. 重组胶质细胞源性神经营养因子腺病毒保护小鼠中脑多巴胺能神经元[J]. 北京大学学报(医学版), 2003, 35(3): 256-260. |
| [5] | 何其华, 周慧芳, 薛冰, 牛东滨, 王晓民. 雷公藤单体T10对谷氨酸所致PC12细胞损伤的保护作用及机制研究[J]. 北京大学学报(医学版), 2003, 35(3): 252-255. |
| [6] | 李凌松, 路艳艳. 神经干细胞及帕金森病的细胞治疗[J]. 北京大学学报(医学版), 2002, 34(5): 499-505. |
|
||