北京大学学报(医学版) ›› 2021, Vol. 53 ›› Issue (2): 425-433. doi: 10.19723/j.issn.1671-167X.2021.02.033
冷冻电镜成像中噪声的滤波方法进展
- 1.北京大学基础医学院生物化学与生物物理学系,北京 100191
2.北京大学医学部医学技术研究院,北京 100191
Progress in filters for denoising cryo-electron microscopy images
HUANG Xin-rui1,LI Sha2,GAO Song2,Δ( )
)
- 1. Department of Biochemistry and Biophysics, Peking University School of Basic Medical Sciences, Beijing 100191, China
2. Institute of Medical Technology, Peking University Health Science Center, Beijing 100191, China
中图分类号:
- R312
| [1] |
Bajaj C, Goswami S, Zhang Q. Detection of secondary and supersecondary structures of proteins from cryo-electron microscopy[J]. J Struct Biol, 2012,177(2):367-381.
pmid: 22186625 |
| [2] | Merino F, Raunser S. Electron cryo-microscopy as a tool for structure-based drug development[J]. Angew Chem Int Edit, 2017,56(11):2846-2860. |
| [3] | Armbruster BL, Kawasaki M, Kersker M, et al. Advanced instrumentation for high resolution transmission cryo-electron microscopy[J]. Biophys J, 2002,82(1):489a. |
| [4] |
Zhang X, Zhou ZH. Limiting factors in atomic resolution cryo electron microscopy: No simple tricks[J]. J Struct Biol, 2011,175(3):253-263.
doi: 10.1016/j.jsb.2011.05.004 pmid: 21627992 |
| [5] | Frank J. Single-particle cryo-electron microscopy: the path toward atomic resolution[M]. New Jersey: World Scientific, 2018. |
| [6] |
Wagner J, Schaffer M, Fernandez-Busnadiego R. Cryo-electron tomography: the cell biology that came in from the cold[J]. Febs Lett, 2017,591(17):2520-2533.
doi: 10.1002/1873-3468.12757 pmid: 28726246 |
| [7] |
Al-Amoudi A, Chang JJ, Leforestier A, et al. Cryo-electron microscopy of vitreous sections[J]. Embo J, 2014,23(18):3583-3588.
pmid: 15318169 |
| [8] |
Cabra V, Samso M. Do’s and don’ts of cryo-electron microscopy: A primer on sample preparation and high quality data collection for macromolecular 3D reconstruction[J]. J Vis Exp, 2015(95):52311.
pmid: 25651412 |
| [9] | Bert W, Slos D, Leroux O, et al. Cryo-fixation and associated developments in transmission electron microscopy: a cool future for nematology[J]. Nematology, 2016,18(1):1-14. |
| [10] | Mielanczyk L, Matysiak N, Michalski M, et al. Closer to the native state. Critical evaluation of cryo-techniques for transmission electron microscopy: preparation of biological samples[J]. Folia Histochem Cyto, 2014,52(1):1-17. |
| [11] |
Beck M, Baumeister W. Cryo-electron tomography: Can it reveal the molecular sociology of cells in atomic detail?[J]. Trends Cell Biol, 2016,26(11):825-837.
pmid: 27671779 |
| [12] | Schmidt-Krey I, Cheng Y. Electron crystallography of soluble and membrane proteins: methods and protocols[M]. New York: Humana Press, Springer, 2013. |
| [13] |
Cheng Y, Grigorieff N, Penczek PA, et al. A primer to single-particle cryo-electron microscopy[J]. Cell, 2015,161(3):438-449.
pmid: 25910204 |
| [14] |
Dubochet J, Knapek E. Ups and downs in early electron cryo-microscopy[J]. PLoS Biol, 2018,16(4):e2005550.
pmid: 29672565 |
| [15] | Welter K. Nobel Price for Chemistry cryo-electron microscopy: Cool images in 3D[J]. Chem Unserer Zeit, 2017,51(6):366-368. |
| [16] | Mocibob M. Nobel Prize for Chemistry for 2017: cryo-electron microscopy[J]. Kem Ind, 2017,66(10):703-705. |
| [17] | Neumann E, Estrozi LF, Effantin G, et al. The resolution revolution in cryo-electron microscopy[J]. Med Sci (Paris), 2017,33(12):1111-1117. |
| [18] | Chiu W, Downing KH. Editorial overview: Cryo electron microscopy: Exciting advances in CryoEM herald a new era in structural biology[J]. Curr Opin Struc Biol, 2017, 46(8): iv-viii. |
| [19] | Karuppasamy M, Nejadasl FK, Vulovic M, et al. Radiation damage in single-particle cryo-electron microscopy: effects of dose and dose rate[J]. J Synchrotron Radiat, 2011,18(3):398-412. |
| [20] |
Marchin S, Putaux JL, Pignon F, et al. Effects of the environmental factors on the casein micelle structure studied by cryo transmission electron microscopy and small-angle X-ray scattering/ultrasmall-angle X-ray scattering[J]. J Chem Phys, 2007,126(4):045101.
pmid: 17286511 |
| [21] | Wang K, Doerschuk PC. Understanding dynamics of biological macromolecular complexes by estimating a mechanical model via statistical mechanics from cryo electron microscopy images: proceedings of the 2011 IEEE International Symposium on Biomedical Imaging: From Nano to Macro[C]. San Diego, CA: Biomedical Engineering, Cornell University, 2011: 1935-1938. |
| [22] |
Vulovic M, Ravelli RBG, van Vliet LJ, et al. Image formation modeling in cryo-electron microscopy[J]. J Struct Biol, 2013,183(1):19-32.
pmid: 23711417 |
| [23] | Doerschuk PC. Inverse problems for cryo electron microscopy of viruses: Randomly oriented projection images of random 3-D structures in noise[J]. Proc Spie, 2011,7873(4):565-568. |
| [24] |
Penczek PA. Image restoration in cryo-electron microscopy[J]. Methods Enzymol, 2010,482:35-72.
pmid: 20888957 |
| [25] | Lindert S, Stewart PL, Meiler J. Hybrid approaches: applying computational methods in cryo-electron microscopy[J]. Curr Opin Struc Biol, 2009,19(2):218-225. |
| [26] | Hoffmann A, Perrier V, Grudinin S. A novel fast Fourier transform accelerated off-grid exhaustive search method for cryo-electron microscopy fitting[J]. J Appl Crystallogr, 2017,50(4):1036-1047. |
| [27] |
McMullan G, Vinothkumar KR, Henderson R. Thon rings from amorphous ice and implications of beam-induced Brownian motion in single particle electron cryo-microscopy[J]. Ultramicroscopy, 2015,158:26-32.
doi: 10.1016/j.ultramic.2015.05.017 pmid: 26103047 |
| [28] | Jing ZC, Li M. A wavelet based alternative iteration method for the orientation refinement of cryo-electron microscopy 3D reconstruction[J]. Math Model Anal, 2015,20(3):396-408. |
| [29] | Lee J, Zheng YL, Yin Z, et al. Classification of cryo electron microscopy images, noisy tomographic images recorded with unknown projection directions, by simultaneously estimating reconstructions and application to an assembly mutant of cowpea chlorotic mottle virus and portals of the bacteriophage P22[C]// San Diego, CA: Conference on Image Reconstruction from Incomplete Data VI, 2010: 78000R. 1-10. |
| [30] | Zheng YL, Doerschuk PC. Algorithms for sorting and reconstructing heterogeneous nanoscale biological objects from cryo electron microscopy images[C]. 2009 IEEE International Symposium on Biomedical Imaging: From Nano to Macro, 2009: 169-172. |
| [31] | Mielikainen T, Ravantti J. Sinogram denoising of cryo-electron microscopy images[J]. Lect Notes Comput Sc, 2005,3483:1251-1261. |
| [32] |
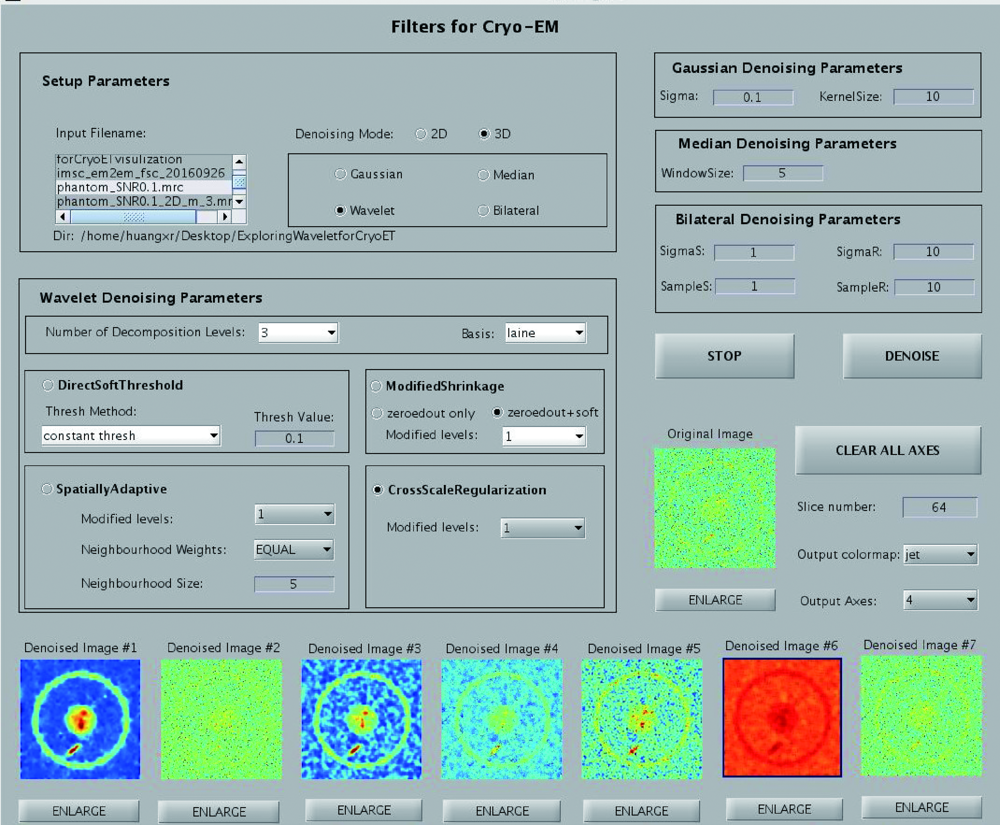
Maiorca M, Hanssen E, Kazmierczak E, et al. Improving the quality of electron tomography image volumes using pre-reconstruction filtering[J]. J Struct Biol, 2012,180(1):132-142.
pmid: 22683346 |
| [33] |
Starosolski Z, Szczepanski M, Wahle M, et al. Developing a denoising filter for electron microscopy and tomography data in the cloud[J]. Biophys Rev, 2012,4(3):223-229.
pmid: 23066432 |
| [34] |
Henderson R. Avoiding the pitfalls of single particle cryo-electron microscopy: Einstein from noise[J]. Proc Natl Acad Sci USA, 2013,110(45):18037-18041.
pmid: 24106306 |
| [35] | Prust CJ, Wang Q, Doerschuk PC, et al. Highly scalable methods for exploiting a label with unknown location in order to orient a set of single-particle cryo electron microscopy images[J]. Proc Spie, 2012,8296(3):4. |
| [36] |
Bhamre T, Zhang T, Singer A. Denoising and covariance estimation of single particle cryo-EM images[J]. J Struct Biol, 2016,195(1):72-81.
pmid: 27129418 |
| [37] |
Sindelar CV, Grigorieff N. Optimal noise reduction in 3D reconstructions of single particles using a volume-normalized filter[J]. J Struct Biol, 2012,180(1):26-38.
pmid: 22613568 |
| [38] | Fernandez-Leiro R, Scheres SHW. A pipeline approach to single-particle processing in RELION[J]. Acta Crystallogr D, 2017,73(Pt6):496-502. |
| [39] |
Campbell MG, Cheng AC, Brilot AF, et al. Movies of ice-embedded particles enhance resolution in electron cryo-microscopy[J]. Structure, 2012,20(11):1823-1828.
pmid: 23022349 |
| [40] | Shigematsu H, Sigworth FJ. Noise models and cryo-EM drift correction with a direct-electron camera[J]. Ultramicroscopy, 2013,131(8):61-69. |
| [41] | Nejadasl FK, Karuppasamy M, Newman ER, et al. Non-rigid image registration to reduce beam-induced blurring of cryo-electron microscopy images[J]. J Synchrotron Radiat, 2013,20(1):58-66. |
| [42] | Brown A, Long F, Nicholls RA, et al. Tools for macromolecular model building and refinement into electron cryo-microscopy reconstructions[J]. Acta Crystallogr D, 2015,71(1):136-153. |
| [43] | Jiang W, Baker ML, Wu Q, et al. Applications of a bilateral denoising filter in biological electron microscopy[J]. J Struct Biol, 2003,144(1):114-122. |
| [44] | Asano S, Engel BD, Baumeister W. In situ cryo-electron tomography: A post-reductionist approach to structural biology[J]. J Mol Biol, 2016,428(2):332-343. |
| [45] |
Evans JE, Hetherington C, Kirkland A, et al. Low-dose aberration corrected cryo-electron microscopy of organic specimens[J]. Ultramicroscopy, 2008,108(12):1636-1644.
doi: 10.1016/j.ultramic.2008.06.004 pmid: 18703285 |
| [46] |
Burger V, Chennubhotla C. Nhs: Network-based hierarchical segmentation for cryo-electron microscopy density maps[J]. Biopolymers, 2012,97(9):732-741.
pmid: 22696408 |
| [47] |
Anderson KL, Page C, Swift MF, et al. Marker-free method for accurate alignment between correlated light, cryo-light, and electron cryo-microscopy data using sample support features[J]. J Struct Biol, 2018,201(1):46-51.
doi: 10.1016/j.jsb.2017.11.001 pmid: 29113849 |
| [48] |
Ali RA, Landsberg MJ, Knauth E, et al. A 3D image filter for parameter-free segmentation of macromolecular structures from electron tomograms[J]. PLoS One, 2012,7(3):e33697.
pmid: 22479430 |
| [49] |
Grange M, Vasishtan D, Grunewald K. Cellular electron cryo tomography and in situ sub-volume averaging reveal the context of microtubule-based processes[J]. J Struct Biol, 2017,197(2):181-190.
pmid: 27374320 |
| [50] |
Wan W, Briggs JAG. Cryo-electron tomography and subtomogram averaging[J]. Methods Enzymol, 2016,579:329-367.
pmid: 27572733 |
| [51] |
Langlois R, Pallesen J, Ash JT, et al. Automated particle picking for low-contrast macromolecules in cryo-electron microscopy[J]. J Struct Biol, 2014,186(1):1-7.
doi: 10.1016/j.jsb.2014.03.001 pmid: 24607413 |
| [52] | Kumar V, Heikkonen J, Engelhardt P, et al. Robust filtering and particle picking in micrograph images towards 3D reconstruction of purified proteins with cryo-electron microscopy[J]. J Struct Biol, 2004,145(1):41-51. |
| [53] |
van der Heide P, Xu XP, Marsh BJ, et al. Efficient automatic noise reduction of electron tomographic reconstructions based on iterative median filtering[J]. J Struct Biol, 2007,158(2):196-204.
doi: 10.1016/j.jsb.2006.10.030 pmid: 17224280 |
| [54] |
Baxter WT, Grassucci RA, Gao HX, et al. Determination of signal-to-noise ratios and spectral SNRs in cryo-EM low-dose imaging of molecules[J]. J Struct Biol, 2009,166(2):126-132.
doi: 10.1016/j.jsb.2009.02.012 pmid: 19269332 |
| [55] |
Sindelar CV, Grigorieff N. An adaptation of the Wiener filter suitable for analyzing images of isolated single particles[J]. J Struct Biol, 2011,176(1):60-74.
pmid: 21757012 |
| [56] |
Pantelic RS, Rothnagel R, Huang CY, et al. The discriminative bilateral filter: An enhanced denoising filter for electron microscopy data[J]. J Struct Biol, 2006,155(3):395-408.
doi: 10.1016/j.jsb.2006.03.030 pmid: 16774838 |
| [57] |
Pantelic RS, Ericksson G, Hamilton N, et al. Bilateral edge filter: Photometrically weighted, discontinuity based edge detection[J]. J Struct Biol, 2007,160(1):93-102.
doi: 10.1016/j.jsb.2007.07.005 pmid: 17822922 |
| [58] |
Wei DY, Yin CC. An optimized locally adaptive non-local means denoising filter for cryo-electron microscopy data[J]. J Struct Biol, 2010,172(3):211-218.
doi: 10.1016/j.jsb.2010.06.021 pmid: 20599508 |
| [59] |
Wang J, Yin CC. A Zernike-moment-based non-local denoising filter for cryo-EM images[J]. Sci China Life Sci, 2013,56(4):384-390.
pmid: 23564187 |
| [60] | Fernandez JJ, Li S. An improved algorithm for anisotropic nonli-near diffusion for denoising cryo-tomograms[J]. J Struct Biol, 2003,144(1):152-161. |
| [61] |
Frangakis AS, Hegerl R. Noise reduction in electron tomographic reconstructions using nonlinear anisotropic diffusion[J]. J Struct Biol, 2001,135(3):239-250.
doi: 10.1006/jsbi.2001.4406 pmid: 11722164 |
| [62] |
Zhong JM, Ning RL. Image denoising based on wavelets and multifractals for singularity detection[J]. IEEE Trans Image Process, 2005,14(10):1435-1447.
doi: 10.1109/tip.2005.849313 pmid: 16238050 |
| [63] | Tian DZ, Ha MH. Applications of wavelet transform in medical image processing[C]// Machine Learning and Cybernetics, 2004. Proceedings of 2004 International Conference on. IEEE, 2004. |
| [64] | Xie GH, Wang YL, Ming L. The application research of wavelet analysis in medical image processing[J]. Wavelet Analysis & Its Applications, 2003(1/2):751-756. |
| [65] | Soumia SA, Messai Z, Ouahabi A, et al. Non parametric denoi-sing methods based on wavelets: Application to electron microscopy images in low exposure time[J]. AIP Conference Proceedings, 2015,1641(1):403-413. |
| [66] | Moss WC, Haase S, Lyle JM, et al. A novel 3D wavelet-based filter for visualizing features in noisy biological data[J]. J Microsc-Oxford, 2005,219(2):43-49. |
| [67] | Huang XR, Li S, Gao S. Applying a modified wavelet shrinkage filter to improve cryo-electron microscopy imaging[J]. J Comput Biol, 2018,25(9):1-9. |
| [68] |
Huang X, Li S, Gao S. Exploring an optimal wavelet-based filter for cryo-ET imaging[J]. Sci Rep, 2018,8(1):2582.
pmid: 29416100 |
| [1] | 邢念增,王明帅,杨飞亚,尹路,韩苏军. 前列腺免活检创新理念的临床实践及其应用前景[J]. 北京大学学报(医学版), 2024, 56(4): 565-566. |
| [2] | 田宇轩,阮明健,刘毅,李德润,吴静云,沈棋,范宇,金杰. 双参数MRI改良PI-RADS评分4分和5分病灶的最大径对临床有意义前列腺癌的预测效果[J]. 北京大学学报(医学版), 2024, 56(4): 567-574. |
| [3] | 唐祖南,胡耒豪,陈震,于尧,章文博,彭歆. 增强现实技术在口腔颌面颈部解剖识别中的应用评价[J]. 北京大学学报(医学版), 2024, 56(3): 541-545. |
| [4] | 吕梁,张铭津,温奧楠,赵一姣,王勇,李晶,杨庚辰,柳大为. 应用三维软组织空间线角模板法评价颏部对称性[J]. 北京大学学报(医学版), 2024, 56(1): 106-110. |
| [5] | 毛渤淳,田雅婧,王雪东,李晶,周彦恒. 骨性Ⅱ类高角患者拔牙矫治前后的面部软硬组织变化[J]. 北京大学学报(医学版), 2024, 56(1): 111-119. |
| [6] | 凌晓彤,屈留洋,郑丹妮,杨静,闫雪冰,柳登高,高岩. 牙源性钙化囊肿与牙源性钙化上皮瘤的三维影像特点[J]. 北京大学学报(医学版), 2024, 56(1): 131-137. |
| [7] | 徐心雨,吴灵,宋凤岐,李自力,张益,刘筱菁. 基于下颌运动轨迹的正颌外科术中下颌骨髁突定位方法及初步精度验证[J]. 北京大学学报(医学版), 2024, 56(1): 57-65. |
| [8] | 李穗,马雯洁,王时敏,丁茜,孙瑶,张磊. 上前牙种植单冠修复体切导的数字化设计正确度[J]. 北京大学学报(医学版), 2024, 56(1): 81-87. |
| [9] | 刘毅,袁昌巍,吴静云,沈棋,肖江喜,赵峥,王霄英,李学松,何志嵩,周利群. 靶向穿刺+6针系统穿刺对PI-RADS 5分患者的前列腺癌诊断效能[J]. 北京大学学报(医学版), 2023, 55(5): 812-817. |
| [10] | 袁昌巍,李德润,李志华,刘毅,山刚志,李学松,周利群. 多参数磁共振成像中动态对比增强状态在诊断PI-RADS 4分前列腺癌中的应用[J]. 北京大学学报(医学版), 2023, 55(5): 838-842. |
| [11] | 刘颖,霍然,徐慧敏,王筝,王涛,袁慧书. 磁共振血管壁成像评估颈动脉中重度狭窄患者斑块特征与脑血流灌注的相关性[J]. 北京大学学报(医学版), 2023, 55(4): 646-651. |
| [12] | 傅强,高冠英,徐雁,林卓华,孙由静,崔立刚. 无症状髋关节前上盂唇撕裂超声与磁共振检查的对比研究[J]. 北京大学学报(医学版), 2023, 55(4): 665-669. |
| [13] | 刘想,谢辉辉,许玉峰,张晓东,陶晓峰,柳林,王霄英. 人工智能对提高放射科住院医生诊断胸部肋骨骨折一致性的价值[J]. 北京大学学报(医学版), 2023, 55(4): 670-675. |
| [14] | 黄莹,吴志远,周行红,蔡志刚,张杰. 股前外侧皮瓣修复上颌骨缺损术后面部软组织对称性感观分级[J]. 北京大学学报(医学版), 2023, 55(4): 708-715. |
| [15] | 张雯,刘筱菁,李自力,张益. 基于解剖标志的鼻翼基底缩窄缝合术对正颌患者术后鼻唇部形态的影响[J]. 北京大学学报(医学版), 2023, 55(4): 736-742. |
|
||