北京大学学报(医学版) ›› 2021, Vol. 53 ›› Issue (6): 1020-1025. doi: 10.19723/j.issn.1671-167X.2021.06.002
类风湿关节炎患者外周血TWEAK基因启动子区甲基化状态及其表达
- 昆明医科大学第一附属医院风湿免疫科,昆明 650032
Methylation status and expression of TWEAK gene promoter region in peripheral blood of patients with rheumatoid arthritis
LOU Xue,LIAO Li,LI Xing-jun,WANG Nan,LIU Shuang,CUI Ruo-mei,XU Jian( )
)
- Department of Rheumatology and Immunology, First Affiliated Hospital of Kunming Medical University, Kunming 650032, China
摘要:
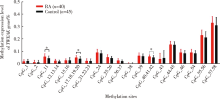
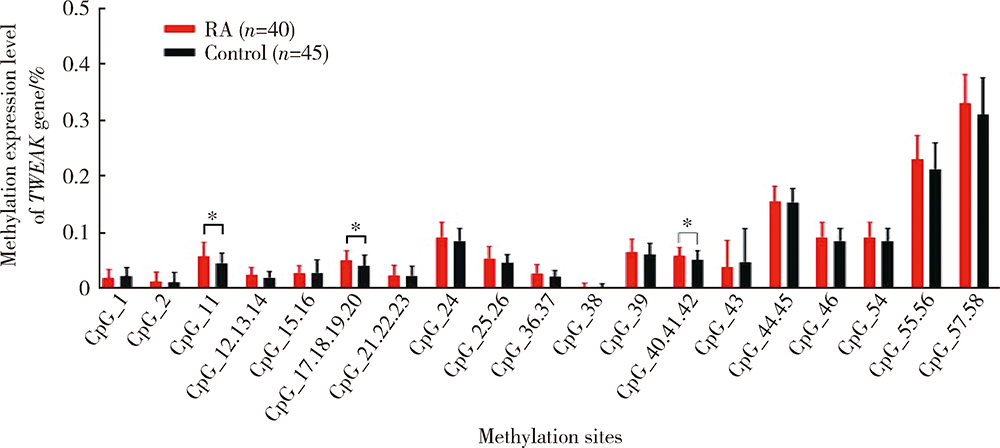
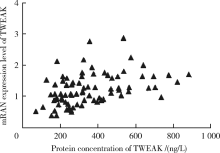
目的:通过检测外周血肿瘤坏死因子样凋亡弱诱导剂(tumor necrosis factor-like weak inducer of apoptosis,TWEAK)基因DNA甲基化水平、mRNA表达水平及血清蛋白浓度,探究TWEAK基因与类风湿关节炎(rheumatoid arthritis,RA)发病机制的关联。方法:采用MassARRAY法检测112例RA患者和86例匹配的健康志愿者外周血TWEAK基因DNA甲基化水平,采用实时荧光定量PCR法检测外周血TWEAK基因mRNA表达水平,采用酶联免疫吸附测定法检测血清TWEAK蛋白浓度。比较RA组和健康对照组TWEAK基因DNA甲基化水平、mRNA表达水平及血清蛋白浓度,并分析其与疾病活动度的关系。结果:RA组TWEAK基因总体甲基化水平和CpG_11、CpG_17.18.19.20、CpG_40.41.42位点甲基化水平高于健康对照组(P=0.002,P=0.01,P=0.006,P=0.002),高疾病活动度组CpG_55.56位点甲基化水平高于中低疾病活动度组(P=0.041)。RA组外周血TWEAK基因mRNA表达水平低于健康对照组(P=0.023),高疾病活动度组TWEAK基因mRNA表达水平低于中低疾病活动度组(P=0.035)。RA组血清TWEAK蛋白浓度与健康对照组差异无统计学意义(P=0.508), 但其与mRNA表达水平呈正相关(r=0.482,P<0.001)。结论:TWEAK基因与RA的发病和病情活动程度密切相关,其高甲基化状态可能为调控mRNA低表达的表观遗传学机制之一,可作为临床监测和评价RA病情的重要指标之一。
中图分类号:
- R593.22
| [1] |
Firestein GS. Evolving concepts of rheumatoid arthritis[J]. Nature, 2003, 423(6937):356-361.
doi: 10.1038/nature01661 |
| [2] | Sánchez-Ramón S, López-Longo FJ, Carreño L. Interleukins network in rheumatoid arthritis pathophysiology: Beyond proinflammatory cytokines[J]. Reumatol Clin, 2011, 6(S3):S20-S24. |
| [3] |
Song X, Lin Q. Genomics, transcriptomics and proteomics to elucidate the pathogenesis of rheumatoid arthritis[J]. Rheumatol Int, 2017, 37(8):1257-1265.
doi: 10.1007/s00296-017-3732-3 |
| [4] |
Karami J, Aslani S, Jamshidi A, et al. Genetic implications in the pathogenesis of rheumatoid arthritis: An updated review[J]. Gene, 2019, 702:8-16.
doi: S0378-1119(19)30290-2 pmid: 30904715 |
| [5] |
Park J, Kwok S, Lim M, et al. TWEAK promotes osteoclastogenesis in rheumatoid arthritis[J]. Am J Pathol, 2013, 183(3):857-867.
doi: 10.1016/j.ajpath.2013.05.027 |
| [6] |
Chicheportiche Y, Chicheportiche R, Sizing I, et al. Proinflammatory activity of TWEAK on human dermal fibroblasts and synoviocytes: Blocking and enhancing effects of anti-TWEAK monoclonal antibodies[J]. Arthritis Res, 2002, 4(2):126-133.
pmid: 11879548 |
| [7] |
Karami J, Aslani S, Tahmasebi MN, et al. Epigenetics in rheumatoid arthritis; fibroblast-like synoviocytes as an emerging paradigm in the pathogenesis of the disease[J]. Immunol Cell Biol, 2020, 98(3):171-186.
doi: 10.1111/imcb.12311 pmid: 31856314 |
| [8] |
Singer BD. A practical guide to the measurement and analysis of DNA methylation[J]. Am J Respir Cell Mol Biol, 2019, 61(4):417-428.
doi: 10.1165/rcmb.2019-0150TR |
| [9] |
Hua XM, Wang J, Qian DM, et al. DNA methylation level of promoter region of activating transcription factor 5 in glioma[J]. J Zhejiang Univ Sci B, 2015, 16(9):757-762.
doi: 10.1631/jzus.B1500067 |
| [10] |
Fuso A, Raia T, Orticello M, et al. The complex interplay between DNA methylation and miRNAs in gene expression regulation[J]. Biochimie, 2020, 173:12-16.
doi: 10.1016/j.biochi.2020.02.006 |
| [11] | 中华医学会风湿病学分会. 类风湿关节炎诊断及治疗指南[J]. 中华风湿病学杂志, 2010(4):265-270. |
| [12] |
Schwartz N, Su L, Burkly LC, et al. Urinary TWEAK as a biomarker of lupus nephritis: A multicenter cohort study[J]. Arthritis Res Ther, 2009, 11(5):R143.
doi: 10.1186/ar2816 |
| [13] |
Xu W, Zhao Y, Liu Y. Role of the TWEAK/Fn14 pathway in autoimmune diseases[J]. Immunol Res, 2016, 64(1):44-50.
doi: 10.1007/s12026-015-8761-y |
| [14] |
Kamijo S, Nakajima A, Kamata K, et al. Involvement of TWEAK/Fn14 interaction in the synovial inflammation of RA[J]. Rheumatology (Oxford), 2008, 47(4):442-450.
doi: 10.1093/rheumatology/ken006 pmid: 18310134 |
| [15] | Bertin D, Stephan D, Khrestchatisky M, et al. Is TWEAK a biomarker for autoimmune/chronic inflammatory diseases[J]. Front Immunol, 2013(4):489. |
| [16] |
van Kuijk AW, Wijbrandts CA, Vinkenoog M, et al. TWEAK and its receptor Fn14 in the synovium of patients with rheumatoid arthritis compared to psoriatic arthritis and its response to tumour necrosis factor blockade[J]. Ann Rheum Dis, 2010, 69(1):301-304.
doi: 10.1136/ard.2008.090548 pmid: 19147618 |
| [17] |
Park MC, Chung SJ, Jung SJ, et al. Relationship of serum TWEAK level to cytokine level, disease activity, and response to anti-TNF treatment in patients with rheumatoid arthritis[J]. Scand J Rheumatol, 2008, 37(3):173-178.
doi: 10.1080/03009740801898608 pmid: 18465450 |
| [18] |
Dharmapatni A, Smith MD, Crotti TN, et al. TWEAK and Fn14 expression in the pathogenesis of joint inflammation and bone erosion in rheumatoid arthritis[J]. Arthritis Res Ther, 2011, 13(2):R51.
doi: 10.1186/ar3294 |
| [19] |
Maecker H, Varfolomeev E, Kischkel F, et al. TWEAK attenuates the transition from innate to adaptive immunity[J]. Cell, 2005, 123(5):931-944.
doi: 10.1016/j.cell.2005.09.022 |
| [20] |
Guo SC, Zhu Q, Jiang T, et al. Genome-wide DNA methylation patterns in CD4+ T cells from Chinese Han patients with rheumatoid arthritis[J]. Mod Rheumatol, 2017, 27(3):441-447.
doi: 10.1080/14397595.2016.1218595 |
| [21] |
Zhu H, Wu LF, Mo XB, et al. Rheumatoid arthritis-associated DNA methylation sites in peripheral blood mononuclear cells[J]. Ann Rheum Dis, 2019, 78(1):36-42.
doi: 10.1136/annrheumdis-2018-213970 |
| [1] | 刘东武, 陈杰, 高明利, 于静. 类风湿关节炎伴发淋巴结Castleman样病理改变1例[J]. 北京大学学报(医学版), 2024, 56(5): 928-931. |
| [2] | 黄会娜,赵静,赵祥格,白自然,李霞,王冠. 乳酸对类风湿关节炎患者外周血CD4+T细胞亚群的调控作用[J]. 北京大学学报(医学版), 2024, 56(3): 519-525. |
| [3] | 汤晓菲,李永红,丁秋玲,孙卓,张阳,王育梅,田美伊,刘坚. 类风湿关节炎患者下肢深静脉血栓发病率及危险因素[J]. 北京大学学报(医学版), 2024, 56(2): 279-283. |
| [4] | 邹雪,白小娟,张丽卿. 艾拉莫德联合托法替布治疗难治性中重度类风湿关节炎的疗效[J]. 北京大学学报(医学版), 2023, 55(6): 1013-1021. |
| [5] | 吴琦,蔡月明,何娟,黄文蒂,王庆文. 血脂异常与类风湿关节炎肺间质病变的相关性分析[J]. 北京大学学报(医学版), 2023, 55(6): 982-992. |
| [6] | 张警丰,金银姬,魏慧,姚中强,赵金霞. 体重指数与类风湿关节炎临床特征的相关性分析[J]. 北京大学学报(医学版), 2023, 55(6): 993-999. |
| [7] | 金银姬,孙琳,赵金霞,刘湘源. 血清IgA型抗鼠科肉瘤病毒癌基因同源物B1抗体在类风湿关节炎中的意义[J]. 北京大学学报(医学版), 2023, 55(4): 631-635. |
| [8] | 蔡文心,李仕成,刘一鸣,梁如玉,李静,郭建萍,胡凡磊,孙晓麟,李春,刘栩,叶华,邓立宗,李茹,栗占国. 类风湿关节炎临床分层及其特征的横断面研究[J]. 北京大学学报(医学版), 2022, 54(6): 1068-1073. |
| [9] | 程昉,杨邵英,房星星,王璇,赵福涛. CCL28-CCR10通路在类风湿关节炎单核细胞迁移中的作用[J]. 北京大学学报(医学版), 2022, 54(6): 1074-1078. |
| [10] | 刘蕊,赵金霞,闫良. 类风湿关节炎合并下肢静脉血栓患者的临床特点[J]. 北京大学学报(医学版), 2022, 54(6): 1079-1085. |
| [11] | 张警丰,金银姬,魏慧,姚中强,赵金霞. 类风湿关节炎患者生活质量与疾病活动度的横断面研究[J]. 北京大学学报(医学版), 2022, 54(6): 1086-1093. |
| [12] | 高超,陈立红,王莉,姚鸿,黄晓玮,贾语博,刘田. 类风湿关节炎合并纤维肌痛简易分类标准的临床验证[J]. 北京大学学报(医学版), 2022, 54(2): 278-282. |
| [13] | 钟华,徐丽玲,白明欣,苏茵. 类风湿关节炎患者趋化因子CXCL9和CXCL10在骨侵蚀中的作用[J]. 北京大学学报(医学版), 2021, 53(6): 1026-1031. |
| [14] | 罗靓,霍文岗,张钦,李春. 类风湿关节炎合并角膜溃疡的临床特点和相关因素分析[J]. 北京大学学报(医学版), 2021, 53(6): 1032-1036. |
| [15] | 张璐,胡小红,陈澄,蔡月明,王庆文,赵金霞. 类风湿关节炎初治患者颈椎失稳情况及临床特征[J]. 北京大学学报(医学版), 2021, 53(6): 1049-1054. |
|
||