北京大学学报(医学版) ›› 2021, Vol. 53 ›› Issue (6): 1061-1066. doi: 10.19723/j.issn.1671-167X.2021.06.009
肌肉骨骼超声在指导银屑病关节炎临床分型中的价值
- 北京大学第一医院风湿免疫科,北京 100034
Benefit of ultrasound in the phenotype recognition of psoriatic arthritis
SONG Zhi-bo,GENG Yan,DENG Xue-rong,ZHANG Xiao-hui,ZHANG Zhuo-li( )
)
- Department of Rheumatology and Clinical Immunology, Peking University First Hospital, Beijing 100034, China
摘要:
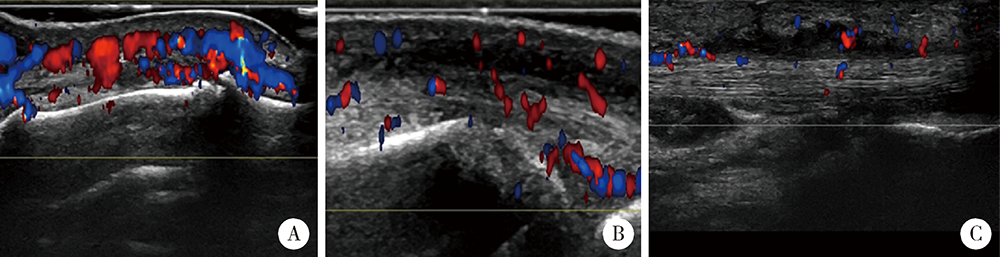
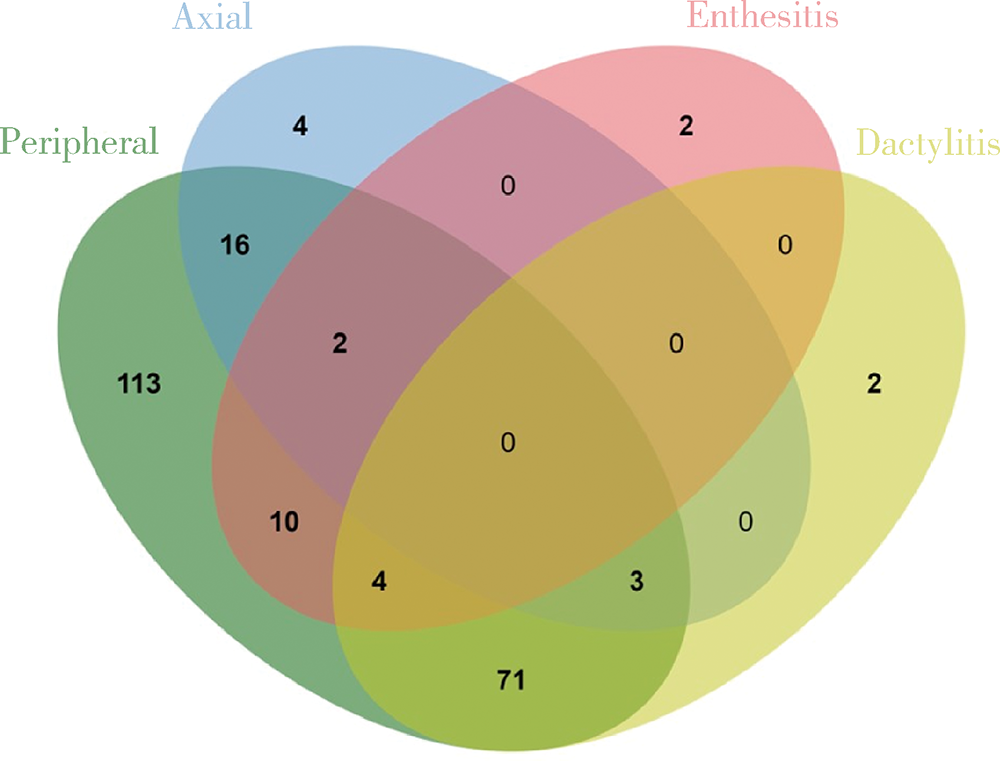
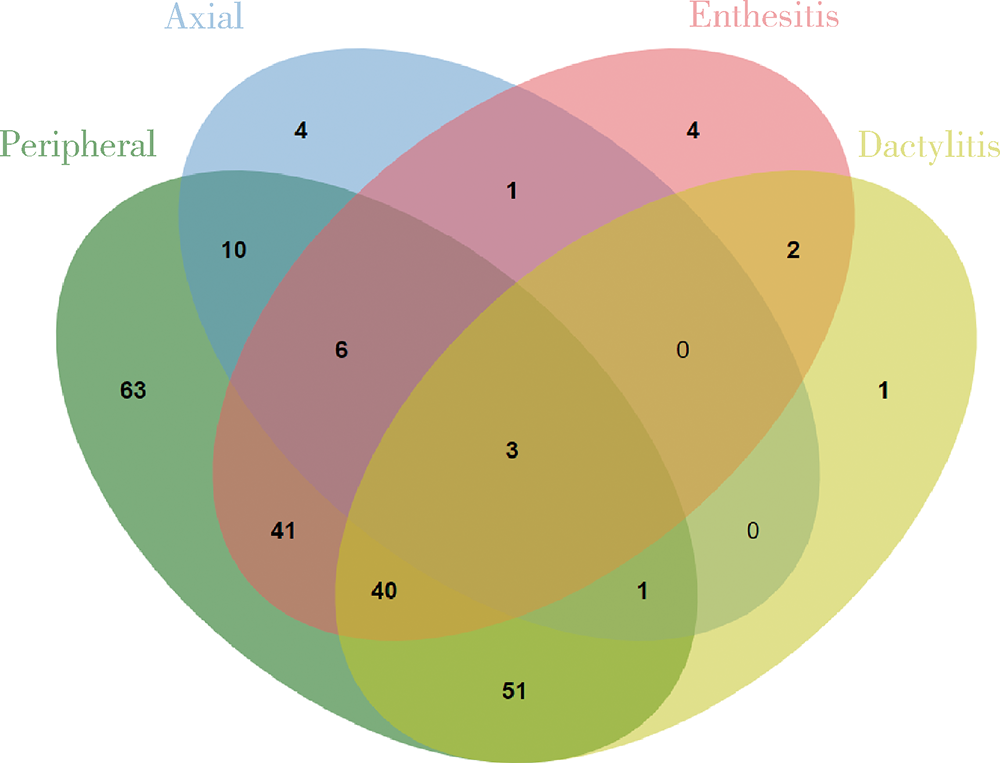
目的:拟探索结合超声检查与单纯根据临床查体两种临床场景下银屑病关节炎(psoriatic arthritis,PsA)患者临床分型的差异。方法:入选2010年1月至2020年10月就诊北京大学第一医院风湿免疫科且临床和超声结果完整的PsA患者,根据患者的临床资料对其进行表型分类,再进一步结合能量多普勒与灰阶超声所发现的附着点炎及指(趾)炎对所有患者进行再次的表型分类,应用韦恩图表示纳入超声前后PsA各临床表型分组,临床表型构成比采用χ2检验或Fisher’s精确检验,纳入超声前后临床表型差异应用Wilcoxon符号秩检验。结果:共纳入227例PsA患者,分别存在一种或多种临床表现。临床查体发现:209(92.1%,209/227)例患者有银屑病皮损,98(43.2%, 98/227)例患者有指(趾)甲病变,219(96.5%,219/227)例患者有外周关节炎表现,25(11.0%,25/227)例患者脊柱受累,80(35.2%,80/227)例患者存在指(趾)炎,18(7.9%,18/227)例患者存在附着点炎。纳入超声评估后,发现另外18例患者超声下有指(趾)炎表现,另外80例患者超声下有急性附着点炎表现,其中异常回声减低55例,肌腱增厚62例,48例可见多普勒血流信号。与单纯根据临床查体分型相比,联合超声检查后对227例患者进行分型,发现最常见的单纯外周关节炎型患者明显减少(49.8% vs. 27.8%,P<0.001), 外周关节炎合并附着点炎患者比例明显增多(4.4% vs. 18.1%,P<0.001),外周关节炎合并附着点炎和指(趾)炎的患者也明显增多(1.8% vs. 17.6%,P<0.001)。结论:超声是发现附着点炎及指(趾)炎的有利工具,借助超声检查可以有效辅助风湿科医生更好地鉴别PsA的病变性质和类型,准确划分临床表型,并进一步指导治疗。
中图分类号:
- R593.22
| [1] |
Ritchlin CT, Colbert RA, Gladman DD. Psoriatic arthritis[J]. N Engl J Med, 2017, 376(10):957-970.
doi: 10.1056/NEJMra1505557 |
| [2] | Parisi R, Iskandar IYK, Kontopantelis E, et al. Global psoriasis atlas. National, regional, and worldwide epidemiology of psoriasis: systematic analysis and modelling study[J]. BMJ, 2020, 369:m1590. |
| [3] | Ogdie A, Weiss P. The epidemiology of psoriatic arthritis[J]. Rheum Dis Clin North Am, 2015, 41(4):545-568. |
| [4] | Coates LC, Kavanaugh A, Mease PJ, et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis 2015 treatment recommendations for psoriatic arthritis[J]. Arthritis Rheumatol, 2016, 68(5):1060-1071. |
| [5] |
Pittam B, Gupta S, Harrison NL, et al. Prevalence of extra-articular manifestations in psoriatic arthritis: a systematic review and meta-analysis[J]. Rheumatology (Oxford), 2020, 59(9):2199-2206.
doi: 10.1093/rheumatology/keaa062 |
| [6] |
Gossec L, Baraliakos X, Kerschbaumer A, et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update[J]. Ann Rheum Dis, 2020, 79(6):700-712.
doi: 10.1136/annrheumdis-2020-217159 pmid: 32434812 |
| [7] | McGonagle D, Benjamin M. Rheumatology[M]. 7th ed. Netherlands: Elsevier, 2018: 1082-1089. |
| [8] |
Alcalde M, Acebes JC, Cruz M, et al. A sonographic enthesitic index of lower limbs is a valuable tool in the assessment of ankylosing spondylitis[J]. Ann Rheum Dis, 2007, 66(8):1015-1019.
pmid: 17158138 |
| [9] |
Felbo SK, Østergaard M, Sørensen IJ, et al. Which ultrasound lesions contribute to dactylitis in psoriatic arthritis and their reliability in a clinical setting[J]. Clin Rheumatol, 2021, 40(3):1061-1067.
doi: 10.1007/s10067-020-05483-9 pmid: 33155158 |
| [10] |
Taylor W, Gladman D, Helliwell P, et al. Classification criteria for psoriatic arthritis: development of new criteria from a large international study[J]. Arthritis Rheumatol, 2006, 54(8):2665-2673.
doi: 10.1002/(ISSN)1529-0131 |
| [11] |
Wakefield RJ, Balint PV, Szkudlarek M, et al. Musculoskeletal ultrasound including definitions for ultrasonographic pathology[J]. J Rheumatol, 2005, 32(12):2485-2487.
pmid: 16331793 |
| [12] |
Balint PV, Terslev L, Aegerter P, et al. Reliability of a consensus-based ultrasound definition and scoring for enthesitis in spondyloarthritis and psoriatic arthritis: an OMERACT US initiative[J]. Ann Rheum Dis, 2018, 77(12):1730-1735.
doi: 10.1136/annrheumdis-2018-213609 pmid: 30076154 |
| [13] |
Tinazzi I, Idolazzi L, Zabotti A, et al. Ultrasonographic detection, definition and quantification of soft tissue oedema in psoriatic dactylitis[J]. Med Ultrason, 2019, 21(4):414-421.
doi: 10.11152/mu-2258 pmid: 31765449 |
| [14] |
Zabotti A, Sakellariou G, Tinazzi I, et al. Novel and reliable dactylitis global sonographic (DACTOS) score in psoriatic arthritis[J]. Ann Rheum Dis, 2020, 79(8):1037-1043.
doi: 10.1136/annrheumdis-2020-217191 pmid: 32430315 |
| [15] |
Bardou P, Mariette J, Escudié F, et al. Jvenn: an interactive venn diagram viewer[J]. BMC Bioinformatics, 2014, 15(1):293.
doi: 10.1186/1471-2105-15-293 |
| [16] |
Coates LC, Moverley AR, McParland L, et al. Effect of tight control of inflammation in early psoriatic arthritis (TICOPA): a UK multicentre, open-label, randomised controlled trial[J]. Lancet, 2015, 386(10012):2489-2498.
doi: 10.1016/S0140-6736(15)00347-5 pmid: 26433318 |
| [17] | Riente L, Carli L, Delle Sedie A. Ultrasound imaging in psoriatic arthritis and ankylosing spondylitis[J]. Clin Exp Rheumatol, 2014, 32(Suppl 80):26-33. |
| [18] |
Helliwell PS. Assessment of enthesitis in psoriatic arthritis[J]. J Rheumatol, 2019, 46(8):869-870.
doi: 10.3899/jrheum.181380 pmid: 31371660 |
| [19] |
Healy PJ, Helliwell PS. Measuring clinical enthesitis in psoriatic arthritis: assessment of existing measures and development of an instrument specific to psoriatic arthritis[J]. Arthritis Rheumatol, 2008, 59(5):686-691.
doi: 10.1002/(ISSN)1529-0131 |
| [20] |
Polachek A, Cook R, Chandran V, et al. The association between sonographic enthesitis and radiographic damage in psoriatic arthritis[J]. Arthritis Res Ther, 2017, 19(1):189.
doi: 10.1186/s13075-017-1399-5 pmid: 28810926 |
| [21] |
Michelsen B, Diamantopoulos AP, Soldal DM, et al. Achilles enthesitis defined by ultrasound is not associated with clinical enthesitis in patients with psoriatic arthritis[J]. RMD Open, 2017, 3(2):e000486.
doi: 10.1136/rmdopen-2017-000486 |
| [22] | Falsetti P, Conticini E, Baldi C, et al. Diffuse peripheral enthesitis in metabolic syndrome: a retrospective clinical and power doppler ultrasound study [J/OL]. Reumatol Clin (Engl Ed), 2021, 2 (2021-02-24) [2021-06-24]. https://pubmed.ncbi.nlm.nih.gov/33640321. |
| [23] |
Bakirci S, Solmaz D, Stephenson W, et al. Entheseal changes in response to age, body mass index, and physical activity: an ultrasound study in healthy people[J]. J Rheumatol, 2020, 47(7):968-972.
doi: 10.3899/jrheum.190540 pmid: 32007938 |
| [24] |
Girolimetto N, Zabotti A, Tinazzi I, et al. Sensitivity to change and clinical correlations of the novel dactylitis global sonographic (DACTOS) score in psoriatic arthritis[J]. Rheumatology (Oxford), 2021, 60(9):4103-4111.
doi: 10.1093/rheumatology/keaa885 |
| [25] |
McGonagle D, Tan AL, Watad A, et al. Pathophysiology, assessment and treatment of psoriatic dactylitis[J]. Nat Rev Rheumatol, 2019, 15(2):113-122.
doi: 10.1038/s41584-018-0147-9 pmid: 30610219 |
| [26] |
Kane D, Greaney T, Bresnihan B, et al. Ultrasonography in the diagnosis and management of psoriatic dactylitis[J]. J Rheumatol, 1999, 26(8):1746-1751.
pmid: 10451072 |
| [27] |
Girolimetto N, Giovannini I, Crepaldi G, et al. Psoriatic dactylitis: current perspectives and new insights in ultrasonography and magnetic resonance imaging[J]. J Clin Med, 2021, 10(12):2604.
doi: 10.3390/jcm10122604 |
| [28] |
Haroon M, Gallagher P, FitzGerald O. Diagnostic delay of more than 6 months contributes to poor radiographic and functional outcome in psoriatic arthritis[J]. Ann Rheum Dis, 2015, 74(6):1045-1050.
doi: 10.1136/annrheumdis-2013-204858 |
| [1] | 耿研,宋志博,张晓慧,邓雪蓉,王昱,张卓莉. 银屑病关节炎抑郁和焦虑患病情况及相关因素[J]. 北京大学学报(医学版), 2020, 52(6): 1048-1055. |
| [2] | 耿研,李伯睿,张卓莉. 系统性红斑狼疮患者有症状关节病变的肌肉骨骼超声特点[J]. 北京大学学报(医学版), 2020, 52(1): 163-168. |
| [3] | 李玉慧,苏波,林福安,费雅楠,于笑霞,范文强,陈海英,张学武,贾园. 银屑病关节炎患者就医行为及治疗现状的多中心调查[J]. 北京大学学报(医学版), 2019, 51(6): 1014-1018. |
| [4] | 代丽怡,巩丹丹,赵金霞. 类风湿因子或抗环瓜氨酸化多肽抗体阳性银屑病关节炎患者的临床特点[J]. 北京大学学报(医学版), 2019, 51(6): 1008-1013. |
|
||