北京大学学报(医学版) ›› 2024, Vol. 56 ›› Issue (6): 963-971. doi: 10.19723/j.issn.1671-167X.2024.06.004
褪黑素调控节律基因表达及其缓解间质性肺纤维化的机制
李炳乐1, 朱凌妍2, 王永福2,*( ), 白力3,4,*(
), 白力3,4,*( )
)
- 1. 内蒙古科技大学包头医学院,内蒙古包头 014010
2. 包头医学院第一附属医院风湿免疫科,内蒙古包头 014010
3. 包头医学院第一附属医院中心实验室,内蒙古包头 014010
4. 内蒙古自体免疫学重点实验室,内蒙古包头 014010
Mechanism of melatonin regulating the expression level of rhythm genes to alleviate interstitial pulmonary fibrosis
Bingle LI1, Lingyan ZHU2, Yongfu WANG2,*( ), Li BAI3,4,*(
), Li BAI3,4,*( )
)
- 1. Baotou Medical College, Inner Mongolia University of Science and Technology, Baotou 014010, Inner Mongolia, China
2. Department of Rheumatology and Immunology, the First Affiliated Hospital of Baotou Medical College, Baotou 014010, Inner Mongolia, China
3. The Central Lab, the First Affiliated Hospital of Baotou Medical College, Baotou 014010, Inner Mongolia, China
4. Inner Mongolia Autoimmune Key Laboratory, Baotou 014010, Inner Mongolia, China
摘要:
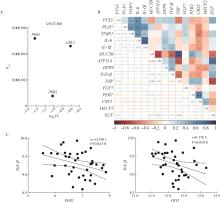
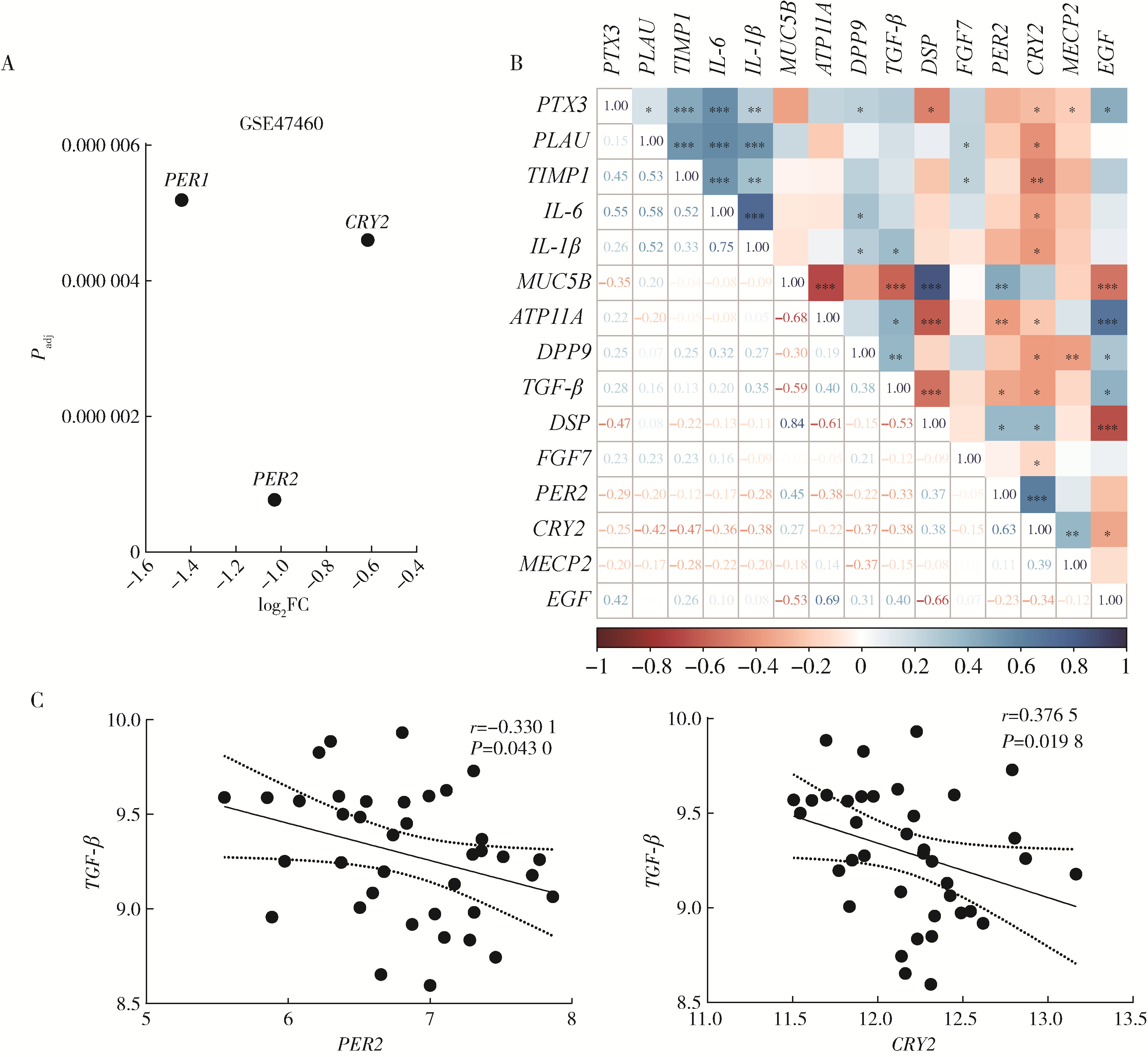
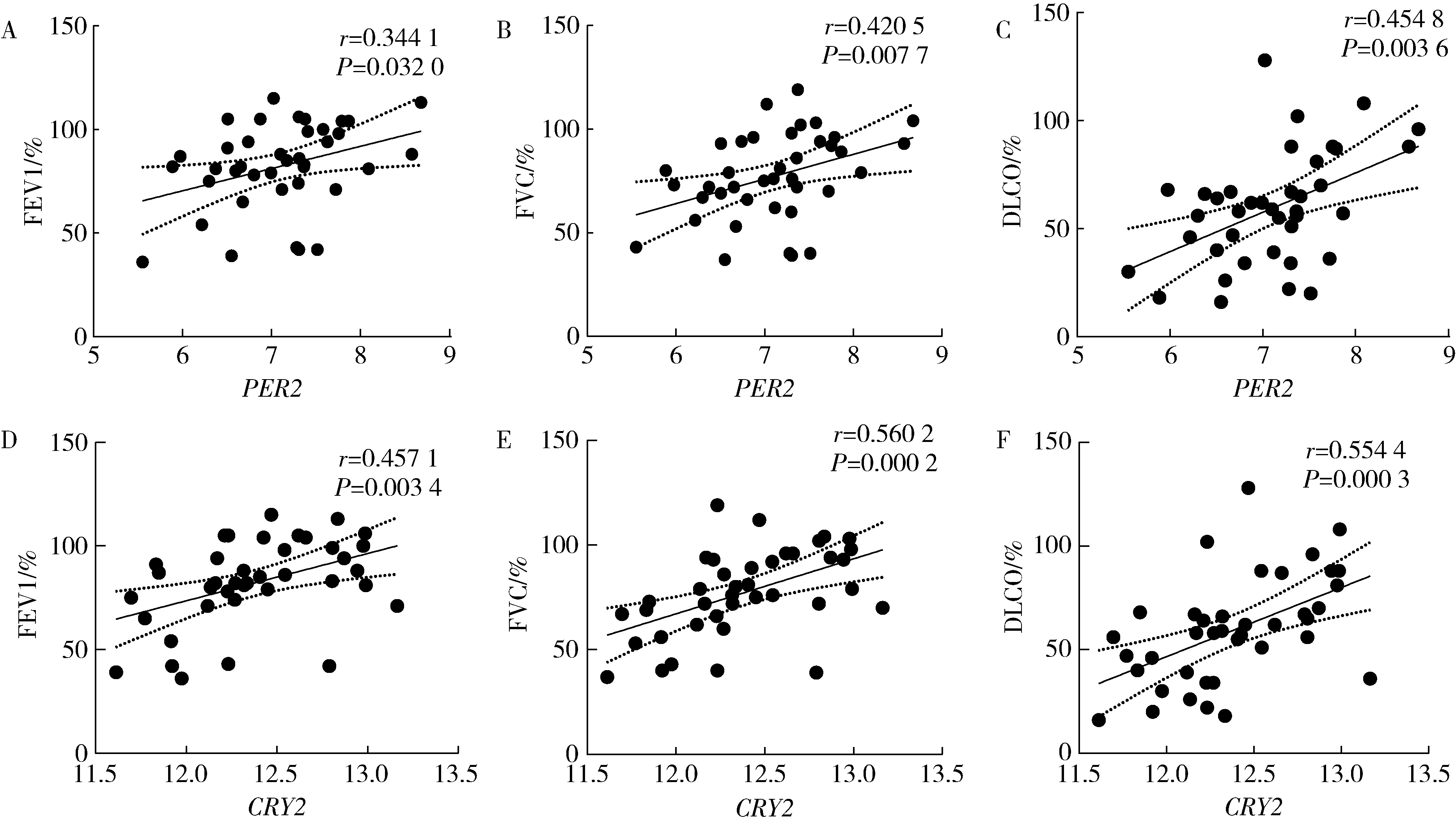
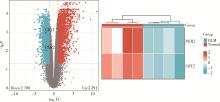
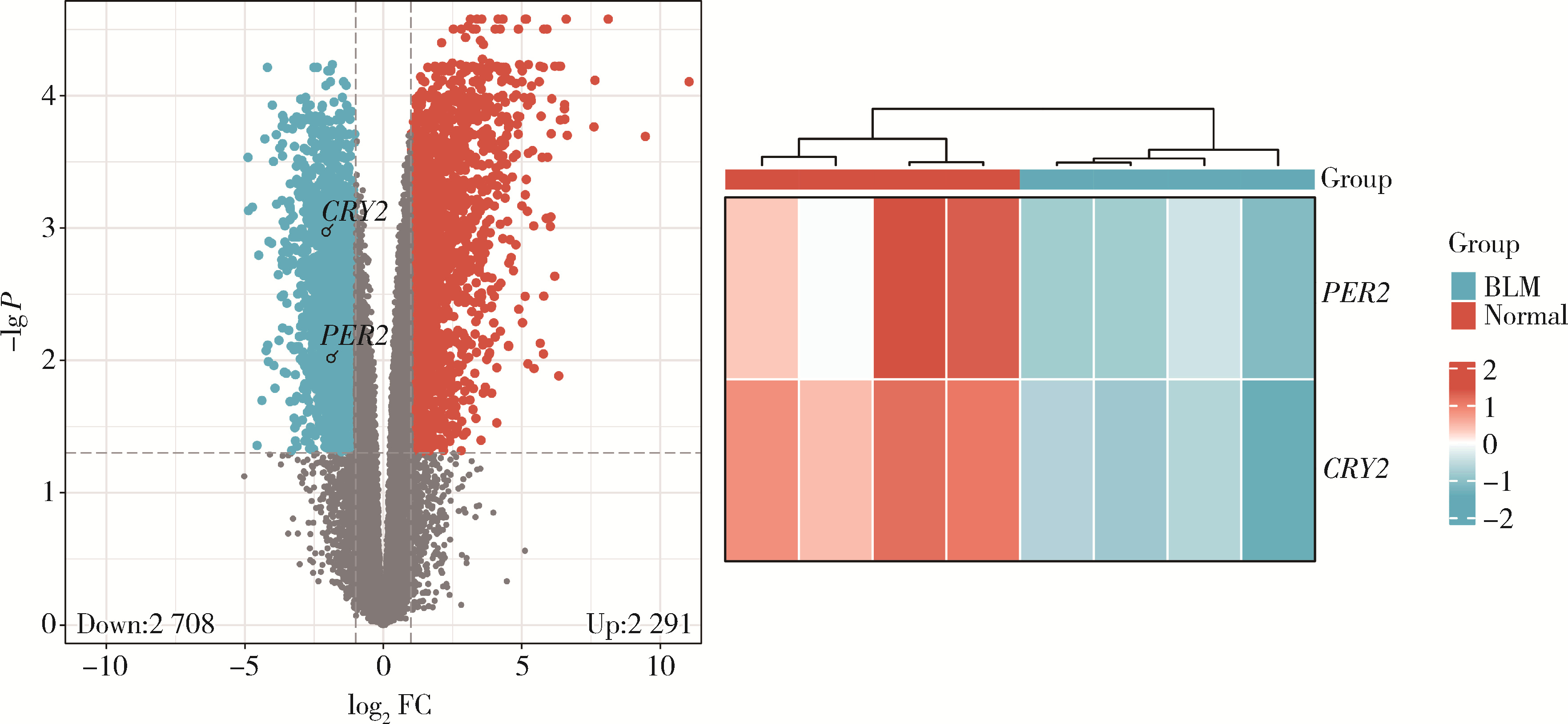
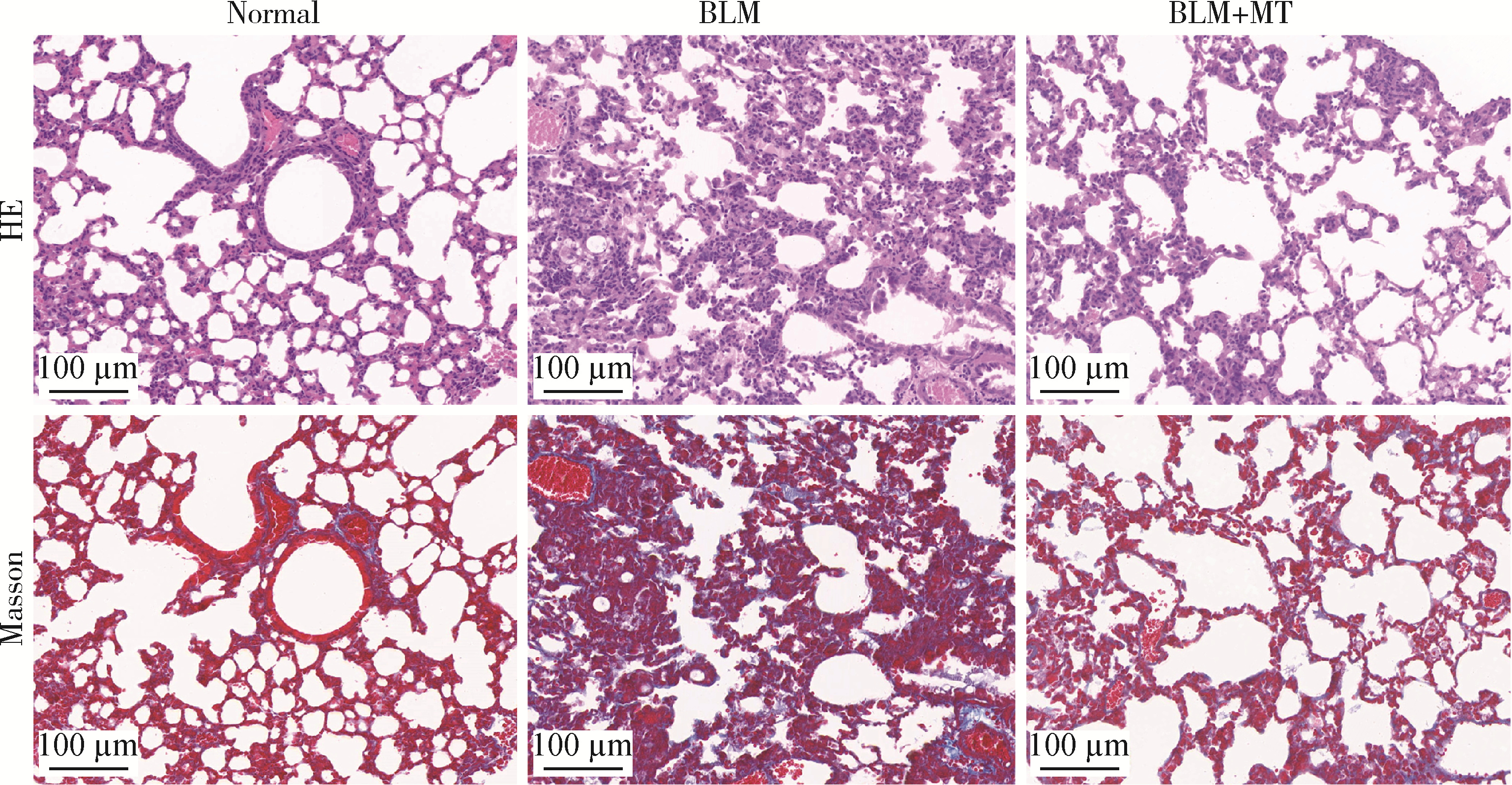
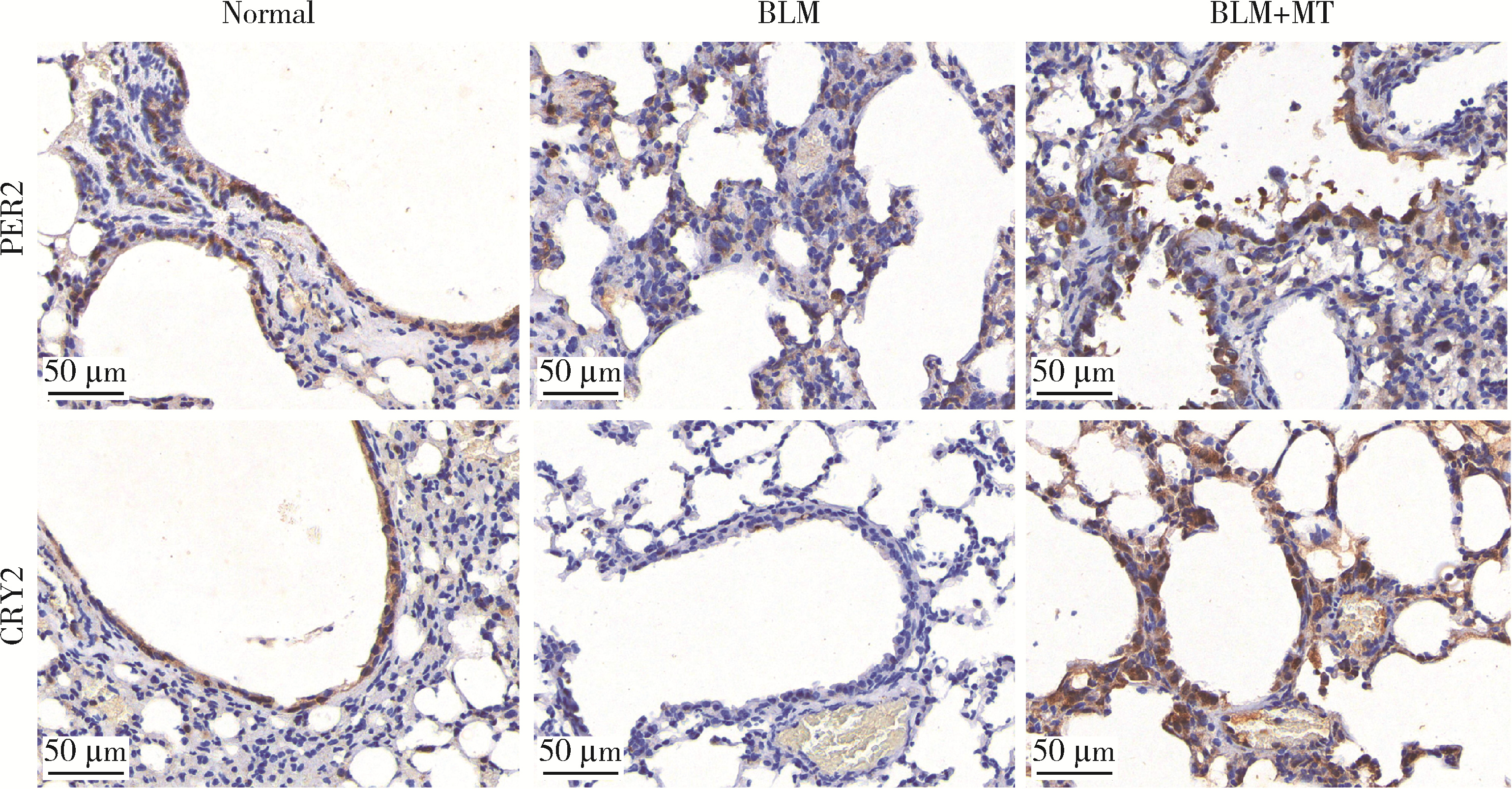
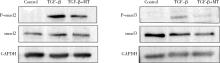
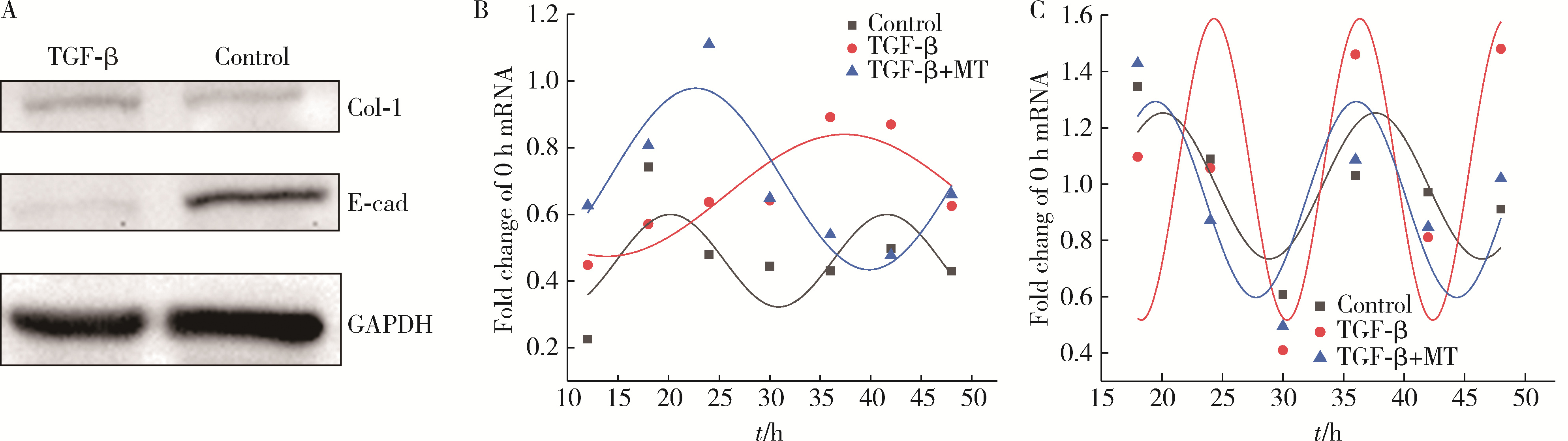
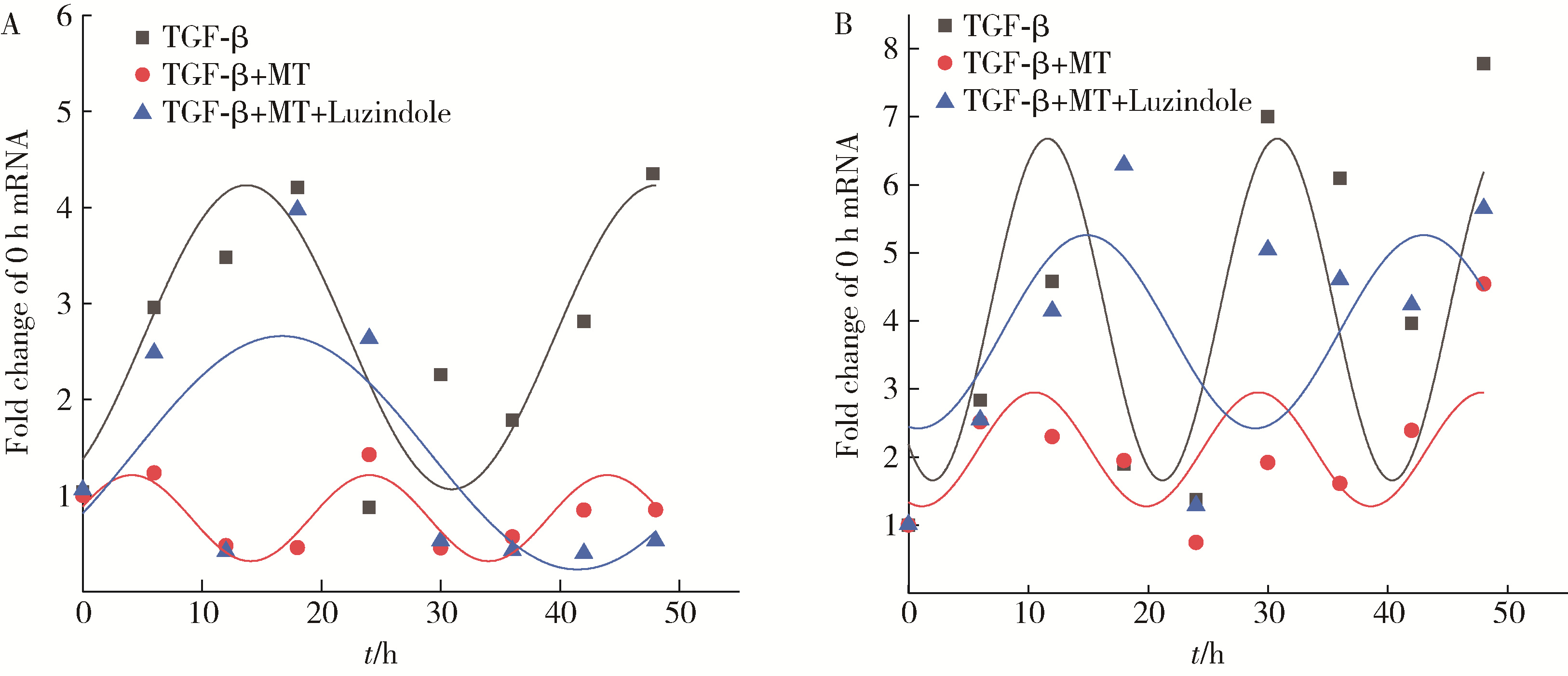
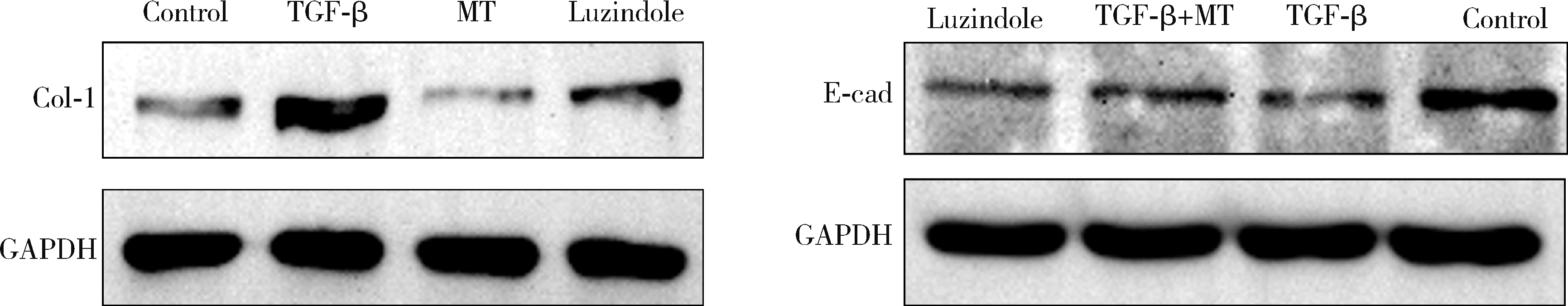
目的: 研究褪黑素(melatonin,MT)干预肺纤维化患者节律基因表达,并分析其缓解肺纤维化疾病进展的机制。方法: 通过高通量基因表达数据库(Gene Expression Omnibus,GEO)筛选肺纤维化患者与健康对照组之间差异表达的生物钟基因,分析节律基因与肺功能及肺纤维化相关基因之间的相关性。构建博来霉素(bleomycin,BLM)诱导小鼠肺纤维化模型,通过测序及免疫组织化学染色观察BLM组以及使用MT干预后(BLM+MT组)肺组织PER2、CRY2表达的差异,通过HE染色及Masson染色观察MT对肺纤维化的影响。用Western blot检测转化生长因子β(transforming growth factor β,TGF-β)诱导肺上皮细胞P-smad2/3的表达,采用实时荧光定量逆转录PCR技术,探究对照组、TGF-β组、TGF-β+MT组生物钟基因的节律表达变化,最后通过MT受体阻滞剂luzindole干预TGF-β+MT组的肺上皮细胞,通过Western blot探究MT减轻TGF-β诱导上皮-间质转化的受体依赖性。结果: (1) 通过对GEO数据集的分析发现,节律基因PER2和CRY2与TGF-β的表达存在负相关性,且与患者肺功能指标存在正相关性。(2)小鼠肺组织转录组测序分析发现BLM组PER2和CRY2的表达较正常组明显减少。同时,病理染色结果显示正常组小鼠肺组织结构完整清晰,肺泡间隔薄;BLM组中肺组织出现大量胶原纤维增生,肺泡结构紊乱;与BLM组相比,BLM+MT组胶原纤维增生及炎性细胞浸润减少;免疫组化染色结果提示BLM组PER2和CRY2的表达量比正常组降低,BLM+MT组比BLM组增加。(3)肺上皮细胞体外TGF-β干预实验结果表明,相较于对照组,TGF-β组P-smad2/3表达量增加,MT干预抑制了TGF-β对P-smad2/3的诱导作用,而MT受体阻滞剂干预又逆转了这一现象,说明MT可以抑制TGF-β通路激活,且该过程存在MT受体依赖性。(4)肺上皮细胞48 h节律实验结果显示,TGF-β+MT组中PER2和CRY2的mRNA节律接近24 h,且有向对照组节律恢复的趋势,而在加入MT受体阻断剂后,其节律的时程和振幅均趋向于TGF-β组。结论: MT通过与其受体结合,可以恢复正常生物钟基因PER2和CRY2的周期性表达,进而抑制TGF-β经典通路的激活,抑制肺纤维化上皮-间质转化病理进程。这一发现为肺纤维化的治疗提供了新的分子靶点和潜在的治疗策略。
中图分类号:
- R563
| 1 |
Koike N , Yoo SH , Huang HC , et al. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals[J]. Science, 2012, 338 (6105): 349- 354.
doi: 10.1126/science.1226339 |
| 2 |
Yamazaki S , Numano R , Abe M , et al. Resetting central and peripheral circadian oscillators in transgenic rats[J]. Science, 2000, 288 (5466): 682- 685.
doi: 10.1126/science.288.5466.682 |
| 3 |
Cox KH , Takahashi JS . Circadian clock genes and the transcriptional architecture of the clock mechanism[J]. J Mol Endocrinol, 2019, 63 (4): R93- R102.
doi: 10.1530/JME-19-0153 |
| 4 |
Fukuhara C , Tosini G . Peripheral circadian oscillators and their rhythmic regulation[J]. Front Biosci, 2003, 8, d642- d651.
doi: 10.2741/1042 |
| 5 | Oishi A , Jockers R . Measuring protein-protein interactions of melatonin receptors by bioluminescence resonance energy transfer (BRET)[J]. Methods Mol Biol, 2022, 2550, 207- 218. |
| 6 |
Cipolla-Neto J , Amaral FGD . Melatonin as a hormone: New phy-siological and clinical insights[J]. Endocr Rev, 2018, 39 (6): 990- 1028.
doi: 10.1210/er.2018-00084 |
| 7 |
Kandalepas PC , Mitchell JW , Gillette MU . Melatonin signal transduction pathways require E-box-mediated transcription of Per1 and Per2 to reset the SCN Clock at dusk[J]. PLoS One, 2016, 11 (6): e0157824.
doi: 10.1371/journal.pone.0157824 |
| 8 |
Zhao SQ , Gao Y , Zhang Y , et al. cAMP/PKA/CREB signaling pathway-mediated effects of melatonin receptor genes on clock gene expression in Bactrian camel ovarian granulosa cells[J]. Domest Anim Endocrinol, 2021, 76, 106609.
doi: 10.1016/j.domaniend.2021.106609 |
| 9 |
Sisto M , Ribatti D , Lisi S . Organ fibrosis and autoimmunity: The role of inflammation in TGFbeta-dependent EMT[J]. Biomolecules, 2021, 11 (2): 310.
doi: 10.3390/biom11020310 |
| 10 |
Frangogiannis N . Transforming growth factor-beta in tissue fibrosis[J]. J Exp Med, 2020, 217 (3): e20190103.
doi: 10.1084/jem.20190103 |
| 11 |
Andugulapati SB , Gourishetti K , Tirunavalli SK , et al. Biochanin- A ameliorates pulmonary fibrosis by suppressing the TGF-beta mediated EMT, myofibroblasts differentiation and collagen deposition in in vitro and in vivo systems[J]. Phytomedicine, 2020, 78, 153298.
doi: 10.1016/j.phymed.2020.153298 |
| 12 |
Cunningham PS , Meijer P , Nazgiewicz A , et al. The circadian clock protein REVERBα inhibits pulmonary fibrosis development[J]. Proc Natl Acad Sci USA, 2020, 117 (2): 1139- 1147.
doi: 10.1073/pnas.1912109117 |
| 13 |
Chen SJ , Yu F , Feng X , et al. DEC1 is involved in circadian rhythm disruption-exacerbated pulmonary fibrosis[J]. Cell Commun Signal, 2024, 22 (1): 245.
doi: 10.1186/s12964-024-01614-w |
| 14 |
Dong C , Gongora R , Sosulski ML , et al. Regulation of transforming growth factor-beta1 (TGF-beta1)-induced pro-fibrotic activities by circadian clock gene BMAL1[J]. Respir Res, 2016, 17, 4.
doi: 10.1186/s12931-016-0320-0 |
| 15 |
Thannickal VJ , Toews GB , White ES , et al. Mechanisms of pulmonary fibrosis[J]. Annu Rev Med, 2004, 55, 395- 417.
doi: 10.1146/annurev.med.55.091902.103810 |
| 16 |
Inui N , Sakai S , Kitagawa M . Molecular pathogenesis of pulmonary fibrosis, with focus on pathways related to TGF-beta and the Ubiquitin-Proteasome pathway[J]. Int J Mol Sci, 2021, 22 (11): 6107.
doi: 10.3390/ijms22116107 |
| 17 |
Chanda D , Otoupalova E , Smith SR , et al. Developmental pathways in the pathogenesis of lung fibrosis[J]. Mol Aspects Med, 2019, 65, 56- 69.
doi: 10.1016/j.mam.2018.08.004 |
| 18 |
Rodriguez-Santana C , Lopez-Rodriguez A , Martinez-Ruiz L , et al. The relationship between Clock genes, sirtuin 1, and mitochondrial activity in head and neck squamous cell cancer: Effects of melatonin treatment[J]. Int J Mol Sci, 2023, 24 (19): 15030.
doi: 10.3390/ijms241915030 |
| 19 |
Shin NR , Park JW , Lee IC , et al. Melatonin suppresses fibrotic responses induced by cigarette smoke via downregulation of TGF-beta1[J]. Oncotarget, 2017, 8 (56): 95692- 95703.
doi: 10.18632/oncotarget.21680 |
| 20 |
Li Y , Zhou Y , Zhao C , et al. The circadian clock gene, BMAL1, promotes radiosensitization in nasopharyngeal carcinoma by inhibiting the epithelial-to-mesenchymal transition via the TGF-beta1/Smads/Snail1 axis[J]. Oral Oncol, 2024, 152, 106798.
doi: 10.1016/j.oraloncology.2024.106798 |
| 21 |
Guo SN , Jiang XQ , Chen N , et al. Melatonin regulates circadian clock proteins expression in allergic airway inflammation[J]. Heliyon, 2024, 10 (6): e27471.
doi: 10.1016/j.heliyon.2024.e27471 |
| 22 |
Wan L , Shi XY , Ge WR , et al. The Instigation of the associations between melatonin, Circadian genes, and epileptic spasms in infant rats[J]. Front Neurol, 2020, 11, 497225.
doi: 10.3389/fneur.2020.497225 |
| 23 |
Fernandez-Ortiz M , Sayed RKA , Roman-Montoya Y , et al. Age and chronodisruption in mouse heart: Effect of the NLRP3 Inflammasome and melatonin therapy[J]. Int J Mol Sci, 2022, 23 (12): 6846.
doi: 10.3390/ijms23126846 |
| [1] | 何珊,陈炘,程琦,朱灵江,张培玉,童淑婷,薛静,杜燕. 托法替布通过JAK/STAT3通路抑制肺成纤维细胞向肌成纤维细胞转化[J]. 北京大学学报(医学版), 2024, 56(3): 505-511. |
| [2] | 雷玲 , 钟小宁, 赵铖, 米存东, 李佳荃, 曾晶晶. Th17细胞及相关细胞因子在系统性硬化病小鼠模型中的表达及意义[J]. 北京大学学报(医学版), 2012, 44(2): 259-264. |
| [3] | 李茹, 李霞, 张晓苹, 相晓红, 栗占国. 类风湿关节炎合并肺间质纤维化的临床特点[J]. 北京大学学报(医学版), 2009, 41(6): 674-677. |
| [4] | 林箐, 倪莲芳, 任雅丽, 刘新民. PPARγ和NF-κB在肺纤维化中的表达与意义[J]. 北京大学学报(医学版), 2009, 41(5): 545-547. |
| [5] | 倪莲芳, 张志刚, 卜定方, 刘新民. Ⅱ类主要组织相容性抗原在博来霉素致大鼠纤维化肺组织中的表达[J]. 北京大学学报(医学版), 2008, 40(5): 514-518. |
| [6] | 赵志杰, 刘新民, 周国鹏, 卜定方, 李雪迎, 宗丽丽, 李江. 老龄大鼠肺内基质金属蛋白酶2、9及基质金属蛋白酶组织抑制因子1、2、3表达变化[J]. 北京大学学报(医学版), 2008, 40(1): 101-104. |
| [7] | 李虹, 刘新民, 耿彬, 潘春水, 齐永芬, 吴胜英, 唐朝枢. 新型气体信号分子硫化氢在大鼠肺纤维化发病中的作用[J]. 北京大学学报(医学版), 2006, 38(2): 140-145. |
| [8] | 张彦萍, 马俊义. 肺纤维化支气管肺泡灌洗液凝血及纤溶指标的变化[J]. 北京大学学报(医学版), 2005, 37(5): 516-519. |
| [9] | 聂立功, 李海潮, 阙呈立, 王广发, 马靖, 李楠, 高志东, 徐小元. SARS第二峰的临床表现和处理[J]. 北京大学学报(医学版), 2003, 35(5): 553-555. |
| [10] | 章巍, 刘新民, 李海潮, 张红. 博来霉素致大鼠肺纤维化模型肺组织MMP-2、MMP-9、MT1-MMP及TIMP-1、TIMP-2的表达[J]. 北京大学学报(医学版), 2002, 34(6): 716-721. |
| [11] | 马靖, 何冰, 李楠, 张红. 白细胞介素-18在肺纤维化大鼠肺组织中的表达[J]. 北京大学学报(医学版), 2002, 34(4): 376-379. |
|
||