北京大学学报(医学版) ›› 2019, Vol. 51 ›› Issue (6): 1019-1024. doi: 10.19723/j.issn.1671-167X.2019.06.007
抗类瓜氨酸化抗体在系统性红斑狼疮中的意义
李英妮,相晓红,赵静,李云,孙峰,王红彦,贾汝琳,胡凡磊( )
)
- 北京大学人民医院风湿免疫科,北京 100044
Significance of anti-carbamylated fibrinogen antibodies in systemic lupus erythematosus
Ying-ni LI,Xiao-hong XIANG,Jing ZHAO,Yun LI,Feng SUN,Hong-yan WANG,Ru-lin JIA,Fan-lei HU( )
)
- Department of Rheumatology & Immunology, Peking University People’s Hospital, Beijing 100044, China
摘要:
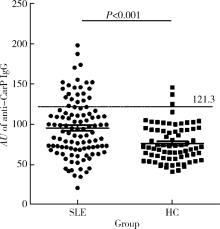
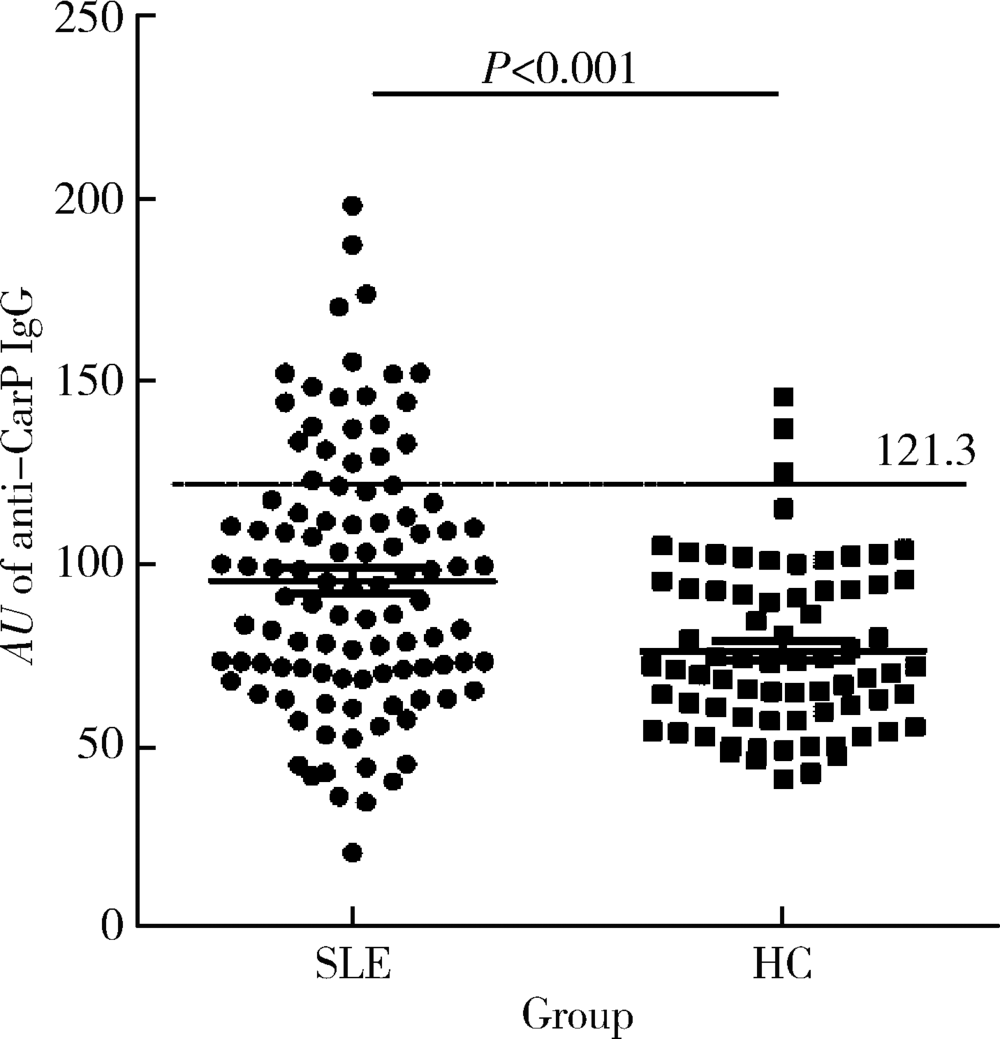
目的 探讨抗类瓜氨酸化抗体(anti-carbamylated fibrinogen antibodies, 抗CarP抗体)在系统性红斑狼疮(systemic lupus erythematosus,SLE)中的意义,SLE是否与类风湿关节炎(rheumatoid arthritis, RA)一样,存在大量针对类瓜氨酸的自身抗体,抗CarP抗体与关节损伤及疾病活动度高度相关。方法 利用酶联免疫吸附法(enzyme-linked immunosorbent assay,ELISA)检测105例确诊的SLE患者和73例健康对照者血清中抗CarP抗体水平,收集其他SLE相关临床和实验室数据,采用SPSS 24.0软件进行相应统计学分析。结果 SLE中存在抗CarP抗体,且明显高于健康对照组(P<0.05)。抗CarP抗体阳性组与阴性组SLE患者相比,在以下临床及实验室指标方面差异有统计学意义:病程、血沉(erythrocyte sedimentation rate,ESR)、C-反应蛋白(C-reactive protein,CRP)、类风湿因子(rheumatoid factor,RF)、抗心磷脂抗体、抗dsDNA抗体、D-二聚体、IgA、IgG、补体C3、补体C4、外周血红细胞计数和血红蛋白水平(P<0.05)。与SLE其他自身抗体相比,抗CarP抗体的阳性率(21.9%)高于抗Sm抗体(15.24%),与抗核糖体P蛋白抗体(22.86%)阳性率相似;在SLE特异性抗体阴性的患者中可检测到抗CarP抗体,其阳性率分别为: 抗Sm(-)组20.2%(18/89),抗dsDNA(-)组9.3%(4/43),抗核小体抗体(-)组12.5%(6/48)和抗核糖体P蛋白抗体(-)组20.9%(17/81)。而且,抗CarP抗体水平与病程、补体C3、补体C4、红细胞及血红蛋白呈明显负相关(P<0.05),与ESR、CRP、IgA、IgG、RF、抗心磷脂抗体、抗dsDNA抗体及D-二聚体呈明显正相关(P<0.05)。结论 SLE患者血清中抗CarP抗体水平升高,抗CarP抗体与SLE患者临床及实验室指标具有相关性。
中图分类号:
- R593.24
| [1] | Lee SJ, Silverman E, Bargman JM . The role of antimalarial agents in the treatment of SLE and lupus nephritis[J]. Nat Rev Nephrol, 2011,7(12):718-729. |
| [2] | Yaniv G, Twig G, Shor DB , et al. A volcanic explosion of autoantibodies in systemic lupus erythematosus: a diversity of 180 different antibodies found in SLE patients[J]. Autoimmun Rev, 2015,14(1):75-79. |
| [3] | Sherer Y, Gorstein A, Fritzler MJ , et al. Autoantibody explosion in systemic lupus erythematosus: more than 100 different anti-bodies found in SLE patients[J]. Semin Arthritis Rheum, 2004,34(2):501-537. |
| [4] | Betancur JF, Gómez-Puerta JA . Antinuclear antibodies mitotic patterns and their clinical associations [J/OL]. Ann Rheum Dis ( 2019-04-29)[2019-08-01]. |
| [5] | Bizzaro N, Villalta D, Giavarina D , et al. Are anti-nucleosome antibodies a better diagnostic marker than anti-dsDNA antibodies for systemic lupus erythematosus? A systematic review and a study of metanalysis[J]. Autoimmun Rev, 2012,12(2):97-106. |
| [6] | Schreier SM, Steinkellner H, Jirovetz L , et al. S-carbamoylation impairs the oxidant scavenging activity of cysteine: its possible impact on increased LDL modification in uraemia[J]. Biochimie, 2011,93(4):772-777. |
| [7] | Gross ML, Piecha G, Bierhaus A , et al. Glycated and carbamylated albumin are more “nephrotoxic” than unmodified albumin in the amphibian kidney[J]. Am J Physiol Renal Physiol, 2011,301(3):476-485. |
| [8] | Trepanier DJ, Thibert RJ . Carbamylation of erythrocyte membrane aminophospholipids: an in vitro and in vivo study[J]. Clin Biochem, 1996,29(4):333-345. |
| [9] | Shi J, Knevel R, Suwannalai P , et al. Autoantibodies recognizing carbamylated proteins are present in sera of patients with rheumatoid arthritis and predict joint damage[J]. Proc Natl Acad Sci USA, 2011,108(42):17372-17377. |
| [10] | Bergum B, Koro C, Delaleu N , et al. Antibodies against carbamylated proteins are present in primary Sjögren’s syndrome and are associated with disease severity[J]. Ann Rheum Dis, 2016,75(8):1494-1500. |
| [11] | Scinocca M, Bell DA, Racapé M , et al. Antihomocitrullinated fibrinogen antibodies are specific to rheumatoid arthritis and frequently bind citrullinated proteins/peptides[J]. J Rheumatol, 2014,41(2):270-279. |
| [12] | López-Hoyos M, Álvarez-Rodríguez L, Mahler M , et al. Anti-carbamylated protein antibodies in patients with ageing associated inflammatory chronic disorders[J]. Rheumatology (Oxford), 2016,55(4):764-766. |
| [13] | Ziegelasch M, van Delft MA, Wallin P , et al. Antibodies against carbamylated proteins and cyclic citrullinated peptides in systemic lupus erythematosus: results from two welldefined European cohorts[J]. Arthritis Res Ther, 2016,18(1):289. |
| [14] | Nakabo S, Yoshifuji H, Hashimoto M , et al. Anti-carbamylated protein antibodies are detectable in various connective tissue dis-eases[J]. J Rheumatol, 2017,44(9):1384-1388. |
| [15] | Hochberg MC . Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus[J]. Arthritis Rheum, 1997,40(9):1725. |
| [16] | Turunen S, Koivula MK, Risteli L , et al. Anticitrulline antibodies can be caused by homocitrulline-containing proteins in rabbits[J]. Arthritis Rheum, 2010,62(11):3345-3352. |
| [17] | Shi J, van de Stadt LA, Levarht EWT , et al. Anti-carbamylated protein (anti-CarP) antibodies precede the onset of rheumatoid arthritis[J]. Ann Rheum Dis, 2014,73(4):780-783. |
| [18] | Ceccarelli F, Perricone C, Colasanti T , et al. Anti-carbamylated protein antibodies as a new biomarker of erosive joint damage in systemic lupus erythematosus[J]. Arthritis Res Ther, 2018,20(1):126 |
| [19] | Ceccarelli F, Perricone C, Cipriano E , et al. Joint involvement in systemic lupus erythematosus: from pathogenesis to clinical assessment[J]. Semin Arthritis Rheum, 2017,47(1):53-64. |
| [20] | Mastrangelo A, Colasanti T, Barbati C , et al. The role of post-translational protein modifications in rheumatological diseases: Focus on rheumatoid arthritis [J/OL]. J Immunol Res, 2015, 2015: 712490(2015-05-18)[2019-08-01]. |
| [1] | 武志慧, 胡明智, 赵巧英, 吕凤凤, 张晶莹, 张伟, 王永福, 孙晓林, 王慧. miR-125b-5p修饰脐带间充质干细胞对系统性红斑狼疮的免疫调控机制[J]. 北京大学学报(医学版), 2024, 56(5): 860-867. |
| [2] | 李正芳,罗采南,武丽君,吴雪,孟新艳,陈晓梅,石亚妹,钟岩. 抗氨基甲酰化蛋白抗体在诊断类风湿关节炎中的应用价值[J]. 北京大学学报(医学版), 2024, 56(4): 729-734. |
| [3] | 乔佳佳,田聪,黄晓波,刘军. 肾结石合并系统性红斑狼疮行经皮肾镜碎石取石术的安全性和有效性评估[J]. 北京大学学报(医学版), 2024, 56(4): 745-749. |
| [4] | 任立敏,赵楚楚,赵义,周惠琼,张莉芸,王友莲,沈凌汛,范文强,李洋,厉小梅,王吉波,程永静,彭嘉婧,赵晓珍,邵苗,李茹. 系统性红斑狼疮低疾病活动度及缓解状况的真实世界研究[J]. 北京大学学报(医学版), 2024, 56(2): 273-278. |
| [5] | 赖展鸿,李嘉辰,贠泽霖,张永刚,张昊,邢晓燕,邵苗,金月波,王乃迪,李依敏,李玉慧,栗占国. 特发性炎性肌病完全临床应答相关因素的单中心真实世界研究[J]. 北京大学学报(医学版), 2024, 56(2): 284-292. |
| [6] | 孟彦宏,陈怡帆,周培茹. CENP-B抗体阳性的原发性干燥综合征患者的临床和免疫学特征[J]. 北京大学学报(医学版), 2023, 55(6): 1088-1096. |
| [7] | 罗芷筠,吴佳佳,宋优,梅春丽,杜戎. 伴神经精神系统病变的系统性红斑狼疮相关巨噬细胞活化综合征2例[J]. 北京大学学报(医学版), 2023, 55(6): 1111-1117. |
| [8] | 姚海红,杨帆,唐素玫,张霞,何菁,贾园. 系统性红斑狼疮及成人Still病合并巨噬细胞活化综合征的临床特点及诊断指标[J]. 北京大学学报(医学版), 2023, 55(6): 966-974. |
| [9] | 赵祥格,刘佳庆,黄会娜,陆智敏,白自然,李霞,祁荆荆. 干扰素-α介导系统性红斑狼疮外周血CD56dimCD57+自然杀伤细胞功能的损伤[J]. 北京大学学报(医学版), 2023, 55(6): 975-981. |
| [10] | 张璐,陈澄,翁梅婷,郑爱萍,苏美玲,王庆文,蔡月明. 狼疮肾炎患者肾小管间质损伤的自身抗体特征[J]. 北京大学学报(医学版), 2022, 54(6): 1094-1098. |
| [11] | 张琳崎,赵静,王红彦,王宗沂,李英妮,汤稷旸,李思莹,曲进锋,赵明威. 抗ENO1抗体与狼疮性视网膜病变的相关性[J]. 北京大学学报(医学版), 2022, 54(6): 1099-1105. |
| [12] | 李敏,侯林卿,金月波,何菁. 系统性红斑狼疮合并视网膜病变的临床及免疫学特点[J]. 北京大学学报(医学版), 2022, 54(6): 1106-1111. |
| [13] | 邵苗,郭惠芳,雷玲彦,赵清,丁艳杰,林进,吴锐,于峰,李玉翠,苗华丽,张莉芸,杜燕,焦瑞英,庞丽霞,龙丽,栗占国,李茹. 短间期小剂量环磷酰胺治疗系统性红斑狼疮耐受性的多中心对照研究[J]. 北京大学学报(医学版), 2022, 54(6): 1112-1116. |
| [14] | 罗澜,邢晓燕,肖云抒,陈珂彦,朱冯赟智,张学武,李玉慧. 抗合成酶综合征合并心脏受累患者的临床及免疫学特征[J]. 北京大学学报(医学版), 2021, 53(6): 1078-1082. |
| [15] | 肖云抒,朱冯赟智,罗澜,邢晓燕,李玉慧,张学武,沈丹华. 88例重叠肌炎的临床及免疫学特征[J]. 北京大学学报(医学版), 2021, 53(6): 1088-1093. |
|
||