北京大学学报(医学版) ›› 2020, Vol. 52 ›› Issue (1): 43-50. doi: 10.19723/j.issn.1671-167X.2020.01.007
根尖牙乳头干细胞摄取外泌体的介导途径
- 北京大学口腔医学院·口腔医院,牙体牙髓科 国家口腔疾病临床医学研究中心 口腔数字化医疗技术和材料国家工程实验室 口腔数字医学北京市重点实验室,北京 100081
Mediated pathways of exosomes uptake by stem cells of apical papilla
Xiao-min GAO,Xiao-ying ZOU( ),Lin YUE(
),Lin YUE( )
)
- Department of Cariology and Endodontology, Peking University School and Hospital of Stomatology & National Clinical research Center for Oral Diseases & National Engineering Laboratory for Digital and Material Technology of Stomatology & Beijing Key Laboratory of Digital Stomatology, Beijing 100081, China
摘要:
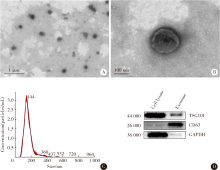
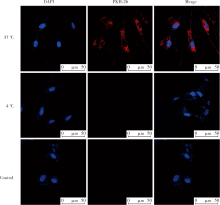
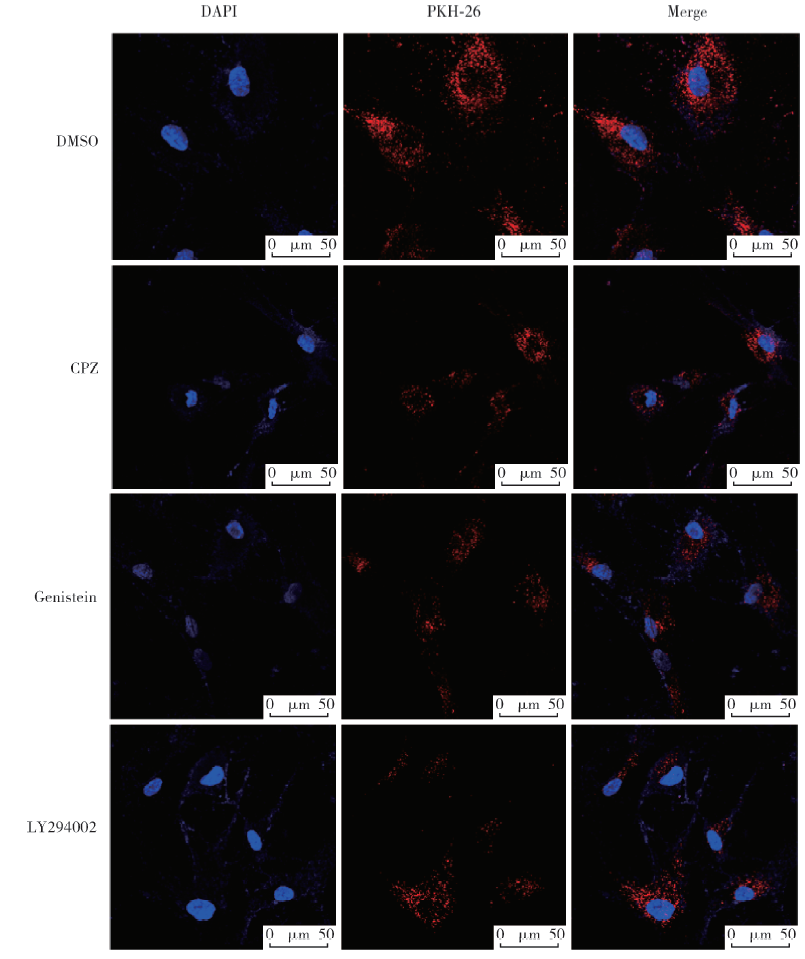
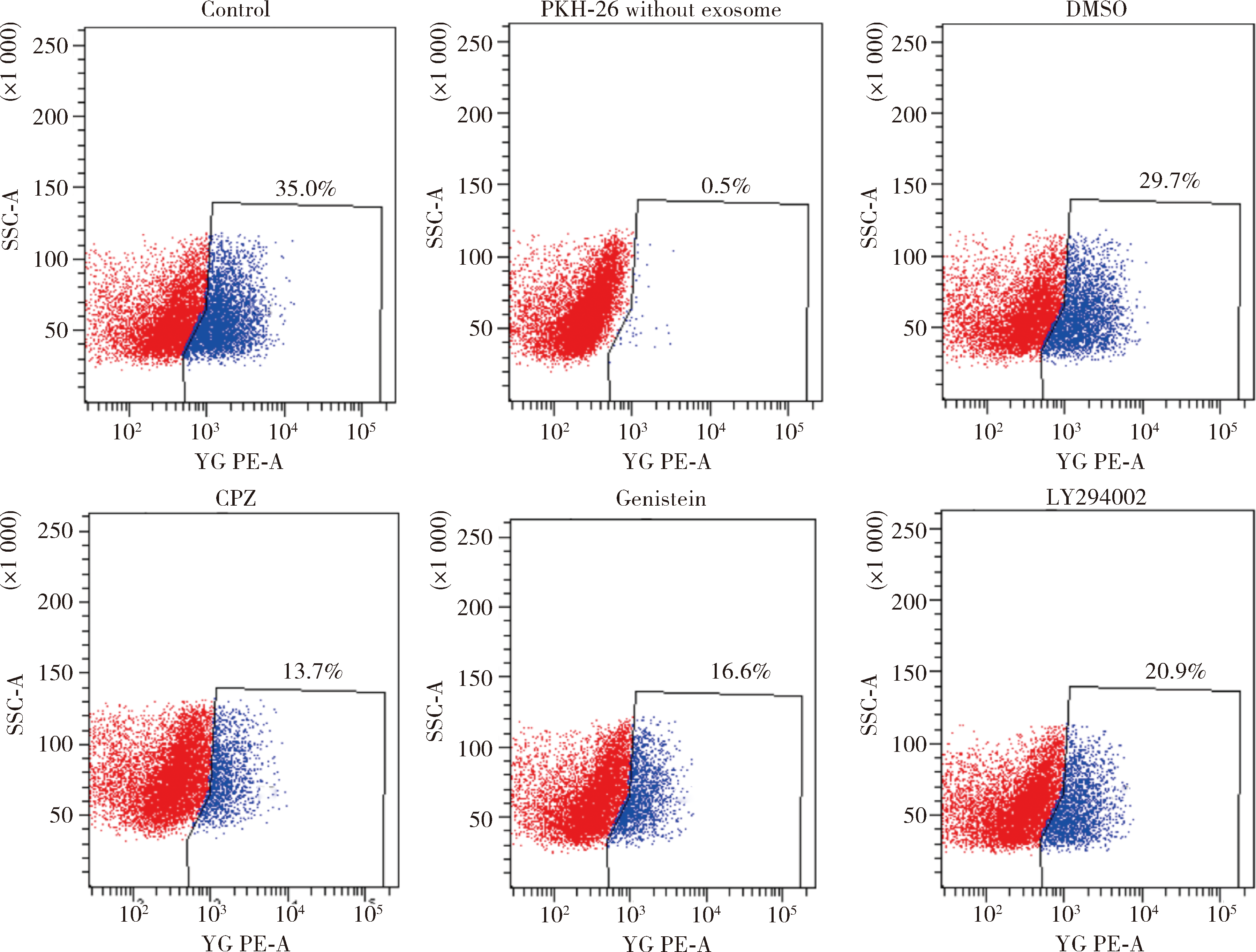
目的:探讨根尖牙乳头干细胞(stem cells from apical papilla, SCAP)摄取牙髓干细胞(dental pulp stem cells, DPSCs)外泌体的作用,为揭示其内吞外泌体的途径提供依据。方法:(1)采用超速离心法结合超滤法提取DPSCs外泌体,采用透射电镜观察法、纳米粒子示踪分析法以及Western Blot对其进行鉴定。(2)采用PKH-26膜标记技术标记DPSCs外泌体,37 ℃条件下将其与SCAP共培养作为阳性对照组,4 ℃条件下将其与SCAP共培养作为低温处理组,同时设置阴性对照组。采用免疫荧光染色法观察不同培养温度条件下SCAP内红色荧光标记情况。(3)通过胞吞抑制方法观察SCAP摄取外泌体的胞吞途径,分别采用10 μmol/L氯丙嗪(chlorpromazine,CPZ,抑制网格蛋白介导的胞吞途径)作为CPZ组、200 μmol /L 金雀异黄素(genistein,抑制小窝蛋白介导的胞吞途径)作为Genistein组、50 μmol /L LY294002抑制巨胞饮(macropinocytosis)作用作为LY294002组处理SCAP,将PKH-26标记的DPSCs外泌体与SCAP共培养,同时设置溶剂对照组(添加与抑制剂组等量的DMSO),采用免疫荧光染色技术观察SCAP内红色荧光标记情况和流式细胞技术分析有红色荧光标记的SCAP百分比。结果:(1)DPSCs外泌体形态呈茶托样,具有双层膜结构,粒径峰值为144 nm,能够表达肿瘤易感基因(tumor susceptibility gene、TSG)101蛋白、CD63蛋白,二者皆为外泌体标志蛋白,符合外泌体特征。(2)免疫荧光结果显示,37 ℃共培养6 h后可见SCAP内有大量红色荧光(PKH-26)标记,而4 ℃共培养6 h后,SCAP内未见明显红色荧光(PKH-26)标记。(3)免疫荧光结果显示胞吞抑制后,SCAP内部红色荧光(PKH-26)标记减少,流式结果显示阳性对照组红色荧光标记的SCAP占35.0%,阴性对照组红色荧光标记的SCAP占0.5%,溶剂对照组红色荧光标记的SCAP占29.7%,CPZ组、Genistein组、LY294002组分别下降至13.7%、 16.6%、 20.9%。结论:SCAP能够摄取DPSCs外泌体,低温可影响该摄取过程;SCAP摄取外泌体主要依赖网格蛋白介导的胞吞途径、小窝蛋白介导的胞吞途径以及巨胞饮途径。
中图分类号:
- R329.2
| [1] | Vlassov AV, Magdaleno S, Setterquist R , et al. Exosomes: Current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials[J]. Biochim Biophys Acta, 2012,1820(7):940-948. |
| [2] | Johnstone RM, Mathew A, Mason AB , et al. Exosome formation during maturation of mammalian and avian reticulocytes: Evidence that exosome release is a major route for externalization of obsolete membrane proteins[J]. J Cell Physiol, 1991,147(1):27-36. |
| [3] | Raposo G, Nijman HW, Stoorvogel W , et al. B lymphocytes secrete antigen-presenting vesicles[J]. J Exp Med, 1996,183(3):1161-1172. |
| [4] | Valadi H, Ekstrom K, Bossios A , et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells[J]. Nat Cell Biol, 2007,9(6):654-672. |
| [5] | Huang CC, Narayanan R, Alapati S , et al. Exosomes as biomimetic tools for stem cell differentiation: Applications in dental pulp tissue regeneration[J]. Biomaterials, 2016,111:103-115. |
| [6] | Xian XH, Gong QM, Li C , et al. Exosomes with highly angioge-nic potential for possible use in pulp regeneration[J]. J Endod, 2018,44(5):751-758. |
| [7] | Liu JY, Chen X, Yue L , et al. CXC chemokine receptor 4 is expressed paravascularly in apical papilla and coordinates with stromal cell-derived factor-1α during transmigration of stem cells from apical papilla[J]. J Endod, 2015,41(9):1430-1436. |
| [8] | 刘敬一, 邹晓英, 陈雪 , 等. 脂多糖对人根尖牙乳头干细胞中基质细胞衍生因子1表达的影响[J]. 中华口腔医学杂志, 2015,50(6):346-351. |
| [9] | Lotvall J, Hill AF, Hochberg F , et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: A position statement from the International Society for Extracellular Vesicles[J]. J Extracell Vesicles, 2014,3:26913. |
| [10] | Pivoraite U, Jarmalaviciute A, Tunaitis V , et al. Exosomes from human dental pulp stem cells suppress carrageenan-induced acute inflammation in mice[J]. Inflammation, 2015,38(5):1933-1941. |
| [11] | Sokolova V, Ludwig AK, Hornung S , et al. Characterisation of exosomes derived from human cells by nanoparticle tracking analysis and scanning electron microscopy[J]. Colloids Surf B Biointerfaces, 2011,87(1):146-150. |
| [12] | Tian T, Zhu YL, Hu FH , et al. Dynamics of exosome internalization and trafficking[J]. J Cell Physiol, 2013,228(7):1487-1495. |
| [13] | He ZL, Liu KZ, Manaloto E , et al. Cold atmospheric plasma induces ATP-dependent endocytosis of nanoparticles and synergistic U373MG cancer cell death[J]. Sci Rep, 2018,8(1):5298. |
| [14] | Kusuma RJ, Manca S, Friemel T , et al. Human vascular endothelial cells transport foreign exosomes from cow’s milk by endocytosis[J]. Am J Physiol Cell Physiol, 2016,310(10):C800-C807. |
| [15] | Tian T, Zhu YL, Zhou YY , et al. Exosome uptake through clathrin-mediated endocytosis and macropinocytosis and mediating miR-21 delivery[J]. J Biol Chem, 2014,289(32):22258-22267. |
| [16] | Horibe S, Tanahashi T, Kawauchi S , et al. Mechanism of reci-pient cell-dependent differences in exosome uptake[J]. Bmc Can-cer, 2018,18(1):47. |
| [1] | 李文根,古晓东,翁锐强,刘苏东,陈超. 血浆外泌体miR-34-5p和miR-142-3p在系统性硬化症中的表达及临床意义[J]. 北京大学学报(医学版), 2023, 55(6): 1022-1027. |
| [2] | 叶雨阳,岳林,邹晓英,王晓燕. 成牙本质方向分化牙髓干细胞外泌体形态及微小RNA表达谱特征[J]. 北京大学学报(医学版), 2023, 55(4): 689-696. |
| [3] | 蒋青,张雨. 新形势下运动损伤特点及细胞生物治疗的应用前景和挑战[J]. 北京大学学报(医学版), 2021, 53(5): 828-831. |
| [4] | 谢静,赵玉鸣,饶南荃,汪晓彤,方滕姣子,李晓霞,翟越,李静芝,葛立宏,王媛媛. 3种口腔颌面部来源的间充质干细胞成血管内皮分化潜能的比较研究[J]. 北京大学学报(医学版), 2019, 51(5): 900-906. |
| [5] | 姚心韵,高晓敏,邹晓英,岳林. 胞内转运对根尖牙乳头干细胞表面CXC趋化因子受体4表达的影响[J]. 北京大学学报(医学版), 2019, 51(5): 893-899. |
| [6] | 汪晓彤,饶南荃,方腾姣子,赵玉鸣,葛立宏. 乳牙牙髓干细胞CD146阳性/阴性细胞亚群生物学特性的比较[J]. 北京大学学报(医学版), 2018, 50(2): 284-292. |
| [7] | 贾维茜,赵玉鸣,葛立宏. 人重组转化生长因子β1促进牙髓干细胞的增殖和矿化[J]. 北京大学学报(医学版), 2017, 49(4): 680-681. |
| [8] | 贺大林,徐珊,郭鹏. 外泌体在泌尿系肿瘤精准诊断中的应用[J]. 北京大学学报(医学版), 2017, 49(4): 561-564. |
| [9] | 杨杰, 赵玉鸣, 王文君, 贾维茜, 葛立宏. 犬根尖牙乳头干细胞的分离培养和生物学特性[J]. 北京大学学报(医学版), 2012, 44(6): 921-926. |
|
||