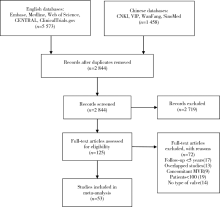
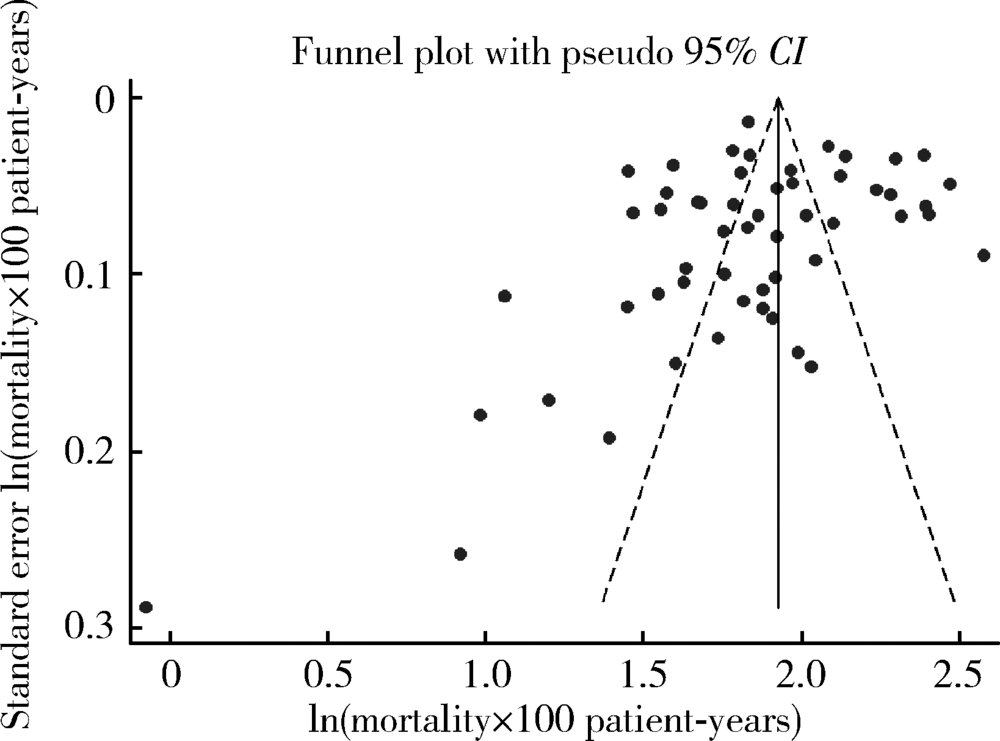
北京大学学报(医学版) ›› 2020, Vol. 52 ›› Issue (3): 547-556. doi: 10.19723/j.issn.1671-167X.2020.03.023
主动脉瓣生物瓣膜安全性的系统评价与meta分析
曾保起1,于树青1,陈瑶1,翟伟2,刘斌2,詹思延1,孙凤1,△( )
)
- 1. 北京大学公共卫生学院流行病与卫生统计学系,北京 100191
2. 北京市药品不良反应中心,北京 100024
Safety of biological valves for aortic valve replacement: A systematic review and meta-analysis
Bao-qi ZENG1,Shu-qing YU1,Yao CHEN1,Wei ZHAI2,Bin LIU2,Si-yan ZHAN1,Feng SUN1,△( )
)
- 1. Department of Epidemiology and Biostatistics, Peking University School of Public Health, Beijing 100191, China
2. Beijing Center for ADR Monitoring, Beijing 100024, China
摘要:
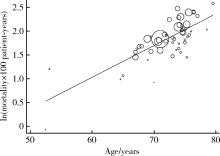
目的 采用系统评价和meta分析的方法评价主动脉瓣置换术中使用生物瓣膜的远期安全性结局。方法 计算机系统检索Medline、Embase、Web of Science、CENTRAL、ClinicalTrial.gov、SinoMed、中国知网、维普和万方数据库在2000年1月1日至2019年1月26日期间发表的随机临床试验、非随机临床试验、队列研究和病例系列研究,纳入标准还包括使用生物瓣膜进行主动脉瓣膜置换术、平均随访时间≥5年、报告了相关安全性结局等。提取研究特征和相关数据,使用Stata 14.0进行meta分析。结果 最终纳入53篇文献共57个研究组,包含47 803名患者。(1)全因死亡率为6.33/100人年(95%CI:5.85~6.84),亚组分析结果显示,猪瓣膜和牛心包瓣膜的死亡率分别为5.69/100人年(95%CI:5.05~6.41)和7.29/100人年(95%CI:6.53~8.13);有支架和无支架瓣膜的死亡率分别为6.69/100人年(95%CI:6.12~7.30)和5.21/100人年(95%CI:4.43~6.14)。(2)血栓栓塞的发生率为1.16/100人年(95%CI:0.96~1.40),心脏起搏器植入率为1.08/100人年(95%CI:0.75~1.54),再手术率为0.77/100人年(95%CI:0.65~0.91),脑卒中的发生率为0.74/100人年(95%CI:0.51~1.06),结构性瓣膜退化的发生率为 0.73/100人年(95%CI:0.59~0.91),大出血的发生率为0.52/100人年(95%CI:0.41~0.65),心内膜炎的发生率为0.38/100人年(95%CI:0.33~0.44),非结构性瓣膜退化的发生率为0.20/100人年(95%CI:0.13~0.31)。结论 生物瓣膜的全因死亡率为6.33/100人年,血栓栓塞、心脏起搏器植入、再手术、脑卒中和结构性瓣膜退化是主要的远期并发症。
中图分类号:
- R654.2
| [1] | Ensminger S, Fujita B, Bauer T, et al. Rapid deployment versus conventional bioprosthetic valve replacement for aortic stenosis[J]. J Am Coll Cardiol, 2018,71(13):1417-1428. |
| [2] | Brown JM, O’Brien SM, Wu C, et al. Isolated aortic valve replacement in North America comprising 108,687 patients in 10 years: changes in risks, valve types, and outcomes in the Society of Thoracic Surgeons National Database[J]. J Thorac Cardiovasc Surg, 2009,137(1):82-90. |
| [3] | Yacoub MH, Takkenberg JJ. Will heart valve tissue engineering change the world?[J]. Nat Clin Pract Cardiovasc Med, 2005,2(2):60-61. |
| [4] | 徐秀林, 张京航, 梁伟, 等. 人工心脏瓣膜质量及安全性评价[J]. 中国医疗器械杂志, 2004,28(5):360-362. |
| [5] | Isaacs AJ, Shuhaiber J, Salemi A, et al. National trends in utilization and in-hospital outcomes of mechanical versus bioprosthetic aortic valve replacements [J]. J Thorac Cardiovasc Surg, 2015, 149(5): 1262-1269.e3. |
| [6] | Dunning J, Gao H, Chambers J, et al. Aortic valve surgery: marked increases in volume and significant decreases in mechanical valve use—an analysis of 41,227 patients over 5 years from the Society for Cardiothoracic Surgery in Great Britain and Ireland National database [J]. J Thorac Cardiovasc Surg, 2011, 142(4): 776-782.e3. |
| [7] |
Siregar S, de Heer F, Groenwold RH, et al. Trends and outcomes of valve surgery: 16-year results of Netherlands Cardiac Surgery National Database[J]. Eur J Cardiothorac Surg, 2014,46(3):386-397.
doi: 10.1093/ejcts/ezu017 pmid: 24554075 |
| [8] | Fujita B, Ensminger S, Bauer T, et al. Trends in practice and outcomes from 2011 to 2015 for surgical aortic valve replacement: an update from the German Aortic Valve Registry on 42776 patients[J]. Eur J Cardiothorac Surg, 2018,53(3):552-559. |
| [9] | Aasbjerg K, Mortensen PE, Norgaard MA, et al. Comparison of survival after aortic valve replacement with mitroflow or perimount prostheses[J]. Semin Thorac Cardiovasc Surg, 2019,31(3):350-358. |
| [10] |
Accola KD, Scott ML, Palmer GJ, et al. Surgical management of aortic valve disease in the elderly: A retrospective comparative study of valve choice using propensity score analysis[J]. J Heart Valve Dis, 2008,17(4):355-365.
pmid: 18751463 |
| [11] |
Albert A, Florath I, Rosendahl U, et al. The late impact of surgical skills and training on the subcoronary implantation of the Freestyle stentless bioprosjournal[J]. J Heart Valve Dis, 2010,19(1):104-114.
pmid: 20329496 |
| [12] | Amabile N, Bical OM, Azmoun A, et al. Long-term results of Freestyle stentless bioprosjournal in the aortic position: a single-center prospective cohort of 500 patients[J]. J Thorac Cardiov Sur, 2014,148(5):1903-1911. |
| [13] |
Andreas M, Wallner S, Ruetzler K, et al. Comparable long-term results for porcine and pericardial prostheses after isolated aortic valve replacement[J]. Eur J Cardiothorac Surg, 2015,48(4):557-561.
doi: 10.1093/ejcts/ezu466 pmid: 25527170 |
| [14] | Anselmi A, Flécher E, Ruggieri VG, et al. Long-term results of the Medtronic Mosaic porcine bioprosjournal in the aortic position[J]. J Thorac Cardiov Sur, 2014,147(6):1884-1891. |
| [15] | Bach DS, Kon ND. Long-term clinical outcomes 15 years after aortic valve replacement with the Freestyle stentless aortic bioprosjournal[J]. Ann Thorac Surg, 2014,97(2):544-551. |
| [16] | Benhameid O, Jamieson WRE, Castella M, et al. CarboMedics mitroflow pericardial aortic bioprosjournal—Performance in patients aged 60 years and older after 15 years[J]. Thorac Cardiov Surg, 2008,56(4):195-199. |
| [17] | Biglioli P, Spampinato N, Cannata A, et al. Long-term outcomes of the Carpentier-Edwards pericardial valve prosjournal in the aortic position: effect of patient age[J]. J Heart Valve Dis, 2004,13(Suppl 1):S49-51. |
| [18] |
Bottio T, Rizzoli G, Thiene G, et al. Hemodynamic and clinical outcomes with the Biocor valve in the aortic position: an 8-year experience[J]. J Thorac Cardiov Sur, 2004,127(6):1616-1623.
doi: 10.1016/j.jtcvs.2003.10.041 |
| [19] |
Bourguignon T, Bouquiaux-Stablo AL, Candolfi P, et al. Very long-term outcomes of the carpentier-edwards perimount valve in aortic position[J]. Ann Thorac Surg, 2015,99(3):831-837.
doi: 10.1016/j.athoracsur.2014.09.030 pmid: 25583467 |
| [20] |
Celiento M, Ravenni G, Tomei L, et al. Excellent durability of the Mosaic porcine aortic bioprosjournal at extended follow up[J]. J Heart Valve Dis, 2018,27(1):97-103.
pmid: 30560605 |
| [21] |
Chan V, Kulik A, Tran A, et al. Long-term clinical and hemodynamic performance of the hancock Ⅱ versus the perimount aortic bioprostheses[J]. Circulation, 2010,122(11 Suppl):S10-16.
doi: 10.1161/CIRCULATIONAHA.109.928085 pmid: 20837899 |
| [22] | Christ T, Grubitzsch H, Claus B, et al. Long-term follow-up after aortic valve replacement with Edwards Prima Plus stentless bioprostheses in patients younger than 60 years of age[J]. J Thorac Cardiov Sur, 2014,147(1):264-269. |
| [23] |
Corbineau H, Verhoye JP, Tauran A, et al. Medtronic intact porcine bioprosjournal in the aortic position: 13-year results[J]. J Heart Valve Dis, 2002,11(4):537-542.
pmid: 12150303 |
| [24] |
David TE, Armstrong S, Maganti M. Hancock Ⅱ bioprosjournal for aortic valve replacement: The gold standard of bioprosthetic valves durability?[J]. Ann Thorac Surg, 2010,90(3):775-781.
doi: 10.1016/j.athoracsur.2010.05.034 |
| [25] |
David TE, Feindel CM, Bos J, et al. Aortic valve replacement with Toronto SPV bioprosjournal: optimal patient survival but suboptimal valve durability[J]. J Thorac Cardiov Sur, 2008,135(1):19-24.
doi: 10.1016/j.jtcvs.2007.04.068 |
| [26] |
de la Fuente A, Sanchez R, Imizcoz A, et al. Intact Medtronic and Carpentier Edwards S.A.V.: clinical and hemodynamic outcomes over 13 years[J]. Cardiovasc Surg, 2003,11(2):139-144.
doi: 10.1016/S0967-2109(03)00010-3 |
| [27] |
Dellgren G, Eriksson MJ, Brodin LÅ, et al. Eleven years’ experience with the Biocor stentless aortic bioprosjournal: Clinical and hemodynamic follow-up with long-term relative survival rate[J]. Eur J Cardiothorac Surg, 2002,22(6):912-921.
doi: 10.1016/s1010-7940(02)00584-5 pmid: 12467813 |
| [28] |
Desai ND, Merin O, Cohen GN, et al. Long-term results of aortic valve replacement with the St. Jude Toronto stentless porcine valve[J]. Ann Thorac Surg, 2004,78(6):2076-2083.
doi: 10.1016/j.athoracsur.2004.05.061 |
| [29] |
Flameng W, Meuris B, Herijgers P, et al. Prosjournal-patient mismatch is not clinically relevant in aortic valve replacement using the Carpentier-Edwards Perimount valve[J]. Ann Thorac Surg, 2006,82(2):530-536.
doi: 10.1016/j.athoracsur.2006.03.089 pmid: 16863756 |
| [30] |
Forcillo J, Pellerin M, Perrault LP, et al. Carpentier-Edwards pericardial valve in the aortic position: 25-years experience[J]. Ann Thorac Surg, 2013,96(2):486-493.
doi: 10.1016/j.athoracsur.2013.03.032 |
| [31] |
Gansera B, Hapfelmeier A, Brandl K, et al. The Mosaic bioprosjournal in the aortic position: 17 years’ results[J]. Thorac Cardiov Surg, 2014,62(1):26-34.
doi: 10.1055/s-0033-1345724 |
| [32] |
Gao G, Wu Y, Grunkemeier GL, et al. Durability of pericardial versus porcine aortic valves[J]. J Am Coll Cardiol, 2004,44(2):384-388.
doi: 10.1016/j.jacc.2004.01.053 pmid: 15261935 |
| [33] |
Glaser N, Franco-Cereceda A, Sartipy U. Late survival after aortic valve replacement with the perimount versus the Mosaic bioprosjournal[J]. Ann Thorac Surg, 2014,97(4):1314-1320.
doi: 10.1016/j.athoracsur.2013.10.078 |
| [34] |
Grunkemeier GL, Furnary AP, Wu Y, et al. Durability of pericardial versus porcine bioprosthetic heart valves[J]. J Thorac Cardiov Sur, 2012,144(6):1381-1386.
doi: 10.1016/j.jtcvs.2012.08.060 |
| [35] |
Guenzinger R, Fiegl K, Wottke M, et al. Twenty-seven-year experience with the St. Jude medical biocor bioprosjournal in the aortic position[J]. Ann Thorac Surg, 2015,100(6):2220-2226.
doi: 10.1016/j.athoracsur.2015.06.027 pmid: 26421496 |
| [36] |
Jamieson WR, Lemieux MD, Sullivan JA, et al. Medtronic intact porcine bioprosjournal experience to twelve years[J]. Ann Thorac Surg, 2001,71(5 Suppl):S278-281.
doi: 10.1016/s0003-4975(01)02548-6 pmid: 11388204 |
| [37] |
Jamieson WRE, Burr LH, Miyagishima RT, et al. Carpentier-Edwards supra-annular aortic porcine bioprosjournal: Clinical perfor-mance over 20 years[J]. J Thorac Cardiov Sur, 2005,130(4):994-1000.
doi: 10.1016/j.jtcvs.2005.03.040 |
| [38] |
Jamieson WRE, Koerfer R, Yankah CA, et al. Mitroflow aortic pericardial bioprosjournal—clinical performance[J]. Eur J Cardiothorac Surg, 2009,36(5):818-824.
doi: 10.1016/j.ejcts.2009.05.020 pmid: 19700338 |
| [39] |
Johnston DR, Soltesz EG, Vakil N, et al. Long-term durability of bioprosthetic aortic valves: implications from 12569 implants[J]. Ann Thorac Surg, 2015,99(4):1239-1247.
doi: 10.1016/j.athoracsur.2014.10.070 pmid: 25662439 |
| [40] | Kurlansky PA, Williams DB, Traad EA, et al. Surgical management of aortic valve disease in elderly patients with and without coronary artery disease: influence on quality of life[J]. J Cardiovasc Surg, 2007,48(2):215-226. |
| [41] |
Lootens L, Verbeke J, Martens T, et al. Ten-year results of aortic valve replacement with first-generation Mitroflow bioprosjournal: Is early degeneration a structural or a technical issue?[J]. Eur J Cardiothorac Surg, 2017,52(2):272-278.
doi: 10.1093/ejcts/ezx117 pmid: 28430883 |
| [42] |
Luciani GB, Santini F, Auriemma S, et al. Long-term results after aortic valve replacement with the Biocor PSB stentless xenograft in the elderly[J]. Ann Thorac Surg, 2001,71(5 Suppl):S306-310.
doi: 10.1016/s0003-4975(01)02525-5 pmid: 11388211 |
| [43] |
McClure RS, McGurk S, Cevasco M, et al. Late outcomes comparison of nonelderly patients with stented bioprosthetic and mechanical valves in the aortic position: a propensity-matched analysis[J]. J Thorac Cardiov Sur, 2014,148(5):1931-1939.
doi: 10.1016/j.jtcvs.2013.12.042 |
| [44] |
Minakata K, Tanaka S, Okawa Y, et al. Long-term outcome of the carpentier-edwards pericardial valve in the aortic position in Japanese patients[J]. Circ J, 2014,78(4):882-889.
doi: 10.1253/circj.CJ-13-1068 |
| [45] |
Minami K, Zittermann A, Schulte-Eistrup S, et al. Mitroflow synergy prostheses for aortic valve replacement: 19 years expe-rience with 1,516 patients[J]. Ann Thorac Surg, 2005,80(5):1699-1705.
doi: 10.1016/j.athoracsur.2005.04.053 pmid: 16242441 |
| [46] |
Mohammadi S, Kalavrouziotis D, Voisine P, et al. Bioprosthetic valve durability after stentless aortic valve replacement: the effect of implantation technique[J]. Ann Thorac Surg, 2014,97(6):2011-2018.
doi: 10.1016/j.athoracsur.2014.02.040 |
| [47] |
Mykén PSU, Bech-Hansen O. A 20-year experience of 1712 patients with the Biocor porcine bioprosjournal[J]. J Thorac Cardiov Sur, 2009,137(1):76-81.
doi: 10.1016/j.jtcvs.2008.05.068 |
| [48] |
Nishida T, Sonoda H, Oishi Y, et al. Long-term results of aortic valve replacement with mechanical prosjournal or carpentier-edwards perimount bioprosjournal in Japanese patients according to age[J]. Circ J, 2014,78(11):2688-2695.
doi: 10.1253/circj.CJ-14-0466 |
| [49] |
Pavoni D, Badano LP, Ius F, et al. Limited long-term durability of the Cryolife O’Brien stentless porcine xenograft valve[J]. Circulation, 2007,116(11 Suppl):I307-313.
doi: 10.1161/CIRCULATIONAHA.107.688564 pmid: 17846322 |
| [50] |
Kappetein AP, Puvimanasinghe JPA, Takkenberg JJM, et al. Predicted patient outcome after aortic valve replacement with Medtro-nic Stentless Freestyle bioprostheses[J]. J Heart Valve Dis, 2007,16(4):423-429.
pmid: 17702369 |
| [51] |
Repossini A, Fischlein T, Santarpino G, et al. Pericardial stentless valve for aortic valve replacement: long-term results[J]. Ann Thorac Surg, 2016,102(6):1956-1965.
doi: 10.1016/j.athoracsur.2016.05.080 pmid: 27544291 |
| [52] | Riess FC, Fradet G, Lavoie A, et al. Long-term outcomes of the Mosaic bioprosjournal[J]. Ann Thorac Surg, 2018,105(3):7763-7769. |
| [53] | Rizzoli G, Mirone S, Ius P, et al. Fifteen-year results with the Hancock Ⅱ valve: A multicenter experience [J]. J Thorac Car-diov Sur, 2006, 132(3): 602-609.e4. |
| [54] |
Ruggieri VG, Flecher E, Anselmi A, et al. Long-term results of the carpentier-edwards supraannular aortic valve prosjournal[J]. Ann Thorac Surg, 2012,94(4):1191-1197.
doi: 10.1016/j.athoracsur.2012.05.003 |
| [55] |
Sjögren J, Gudbjartsson T, Thulin LI. Long-term outcome of the mitroflow pericardial bioprosjournal in the elderly after aortic valve replacement[J]. J Heart Valve Dis, 2006,15(2):197-202.
pmid: 16607900 |
| [56] | Sponga S, Barbera MD, Pavoni D, et al. Ten-year results of the Freedom Solo stentless heart valve: excellent haemodynamics but progressive valve dysfunction in the long term[J]. Interact Cardiov Th, 2017,24(5):663-669. |
| [57] |
Stanger O, Bleuel I, Gisler F, et al. The Freedom Solo pericardial stentless valve: Single-center experience, outcomes, and long-term durability[J]. J Thorac Cardiov Sur, 2015,150(1):70-77.
doi: 10.1016/j.jtcvs.2015.01.060 |
| [58] | Wang Y, Chen S, Shi J, et al. Mid- to long-term outcome comparison of the Medtronic Hancock Ⅱ and bi-leaflet mechanical aortic valve replacement in patients younger than 60 years of age: a propensity-matched analysis[J]. Interact Cardiov Th, 2016,22(3):280-286. |
| [59] |
Webb J, Parkin D, Tøndel K, et al. A comparison of early redo surgery rates in Mosaic porcine and Perimount bovine pericardial valves[J]. Eur J Cardiothorac Surg, 2018,54(4):724-728.
doi: 10.1093/ejcts/ezy113 pmid: 29579171 |
| [60] |
Wilbring M, Alexiou K, Schumann E, et al. Isolated aortic valve replacement in patients with small aortic annulus-a high-risk group on long-term follow-up[J]. Thorac Cardiov Surg, 2013,61(5):379-385.
doi: 10.1055/s-00000085 |
| [61] |
Zibdeh O, Bugg I, Patel S, et al. Randomized trial of the Carpentier-Edwards supra-annular prosjournal versus the Medtronic Mosaic aortic prosjournal: 10-year results[J]. Eur J Cardiothorac Surg, 2018,54(2):281-287.
doi: 10.1093/ejcts/ezx512 pmid: 29401266 |
| [62] |
Head SJ, Celik M, Kappetein AP. Mechanical versus bioprosthe-tic aortic valve replacement[J]. Eur Heart J, 2017,38(28):2183-2191.
doi: 10.1093/eurheartj/ehx141 pmid: 28444168 |
| [63] |
Rodriguez-Gabella T, Voisine P, Puri R, et al. Aortic bioprosthetic valve durability: incidence, mechanisms, predictors, and management of surgical and transcatheter valve degeneration[J]. J Am Coll Cardiol, 2017,70(8):1013-1028.
doi: 10.1016/j.jacc.2017.07.715 pmid: 28818190 |
| [64] |
Schoen FJ, Levy RJ. Calcification of tissue heart valve substitutes: progress toward understanding and prevention[J]. Ann Thorac Surg, 2005,79(3):1072-1080.
doi: 10.1016/j.athoracsur.2004.06.033 pmid: 15734452 |
| [65] |
Siddiqui RF, Abraham JR, Butany J. Bioprosthetic heart valves: modes of failure[J]. Histopathology, 2009,55(2):135-144.
doi: 10.1111/j.1365-2559.2008.03190.x pmid: 19694820 |
| [66] |
Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease[J]. Eur Heart J, 2017,38(36):2739-2791.
doi: 10.1093/eurheartj/ehx391 pmid: 28886619 |
| [67] |
Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines[J]. J Am Coll Cardiol, 2017,70(2):252-289.
doi: 10.1016/j.jacc.2017.03.011 pmid: 28315732 |
| [68] |
Leon MB, Smith CR, Mack MJ, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients[J]. N Engl J Med, 2016,374(17):1609-1620.
doi: 10.1056/NEJMoa1514616 pmid: 27040324 |
| [69] |
Mack MJ, Leon MB, Smith CR, et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial[J]. Lancet, 2015,385(9986):2477-2484.
doi: 10.1016/S0140-6736(15)60308-7 pmid: 25788234 |
| [70] |
Reardon MJ, van Mieghem NM, Popma JJ, et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients[J]. N Engl J Med, 2017,376(14):1321-1331.
pmid: 28304219 |
| [71] |
Karangelis D, Mazine A, Roubelakis A, et al. What is the role of sutureless aortic valves in today’s armamentarium?[J]. Expert Rev Cardiovasc Ther, 2017,15(2):83-91.
doi: 10.1080/14779072.2017.1273108 pmid: 27977305 |
| [1] | 袁昌巍,王盈进,张书杰,沈胜利,段鸿洲. 显微外科手术与血管内栓塞治疗硬脊膜动静脉瘘临床疗效比较的meta分析[J]. 北京大学学报(医学版), 2022, 54(2): 304-314. |
| [2] | 冯菁楠,高乐,孙一鑫,杨继春,邓思危,孙凤,詹思延. Xpert®MTB/RIF对我国人群活动性肺结核和利福平耐药肺结核诊断准确性的meta分析[J]. 北京大学学报(医学版), 2021, 53(2): 320-326. |
| [3] | 张祎然,饶烽,皮伟,张培训,姜保国. 股骨近端防旋髓内钉与动力髋螺钉治疗不稳定型粗隆间骨折的meta分析[J]. 北京大学学报(医学版), 2019, 51(3): 493-500. |
| [4] | 刘爽,郭雨龙,杨静逸,王维,徐健. 间充质干细胞治疗系统性红斑狼疮有效性的meta分析[J]. 北京大学学报(医学版), 2018, 50(6): 1014-1021. |
| [5] | 石慧峰, 张敬旭, 张嵘, 王晓莉. 中国0~6岁儿童孤独症谱系障碍患病率的meta分析[J]. 北京大学学报(医学版), 2017, 49(5): 798-806. |
| [6] | 马燕燕,章晶晶,高雪梅. 不同下颌前伸度口腔矫治器治疗阻塞性睡眠呼吸暂停低通气综合征的系统评价[J]. 北京大学学报(医学版), 2017, 49(4): 691-699. |
| [7] | 李志霞,武珊珊,杨智荣,詹思延,孙凤. 胰高血糖素样肽1受体激动剂类降糖药致2型糖尿病患者鼻咽炎和上呼吸道感染的网状meta分析[J]. 北京大学学报(医学版), 2016, 48(3): 454-459. |
| [8] | 黄元升, 杨智荣, 詹思延. 简单合并模型与双变量模型在诊断试验Meta分析中的使用现状调查[J]. 北京大学学报(医学版), 2015, 47(3): 483-488. |
| [9] | 刘大锦, 冯孟贤, 刘民. 中国未接受抗病毒治疗的人类免疫缺陷病毒/获得性免疫缺陷综合征(HIV/AIDS)人群HIV原发耐药的Meta分析[J]. 北京大学学报(医学版), 2015, 47(3): 474-482. |
| [10] | 武珊珊, 张越伦, 王巍巍, 陈茹, 孙凤, 詹思延 . 耐多药结核病治疗过程中肝损害发生率的Meta分析[J]. 北京大学学报(医学版), 2014, 46(3): 417-423. |
| [11] | 詹思延. 药物流行病学理论和方法的发展[J]. 北京大学学报(医学版), 2013, 45(03): 343-346. |
| [12] | 方振威, 翟所迪, . 阿瑞吡坦防治化疗致恶心呕吐的Meta分析[J]. 北京大学学报(医学版), 2010, 42(6): 756-763. |
| [13] | 詹思延. 如何做一个好的系统综述和Meta分析[J]. 北京大学学报(医学版), 2010, 42(6): 644-647. |
| [14] | 王亚波, 毛宇, 魏强, 吴泰相, 董强. 达泊西汀治疗早泄的有效性系统评价[J]. 北京大学学报(医学版), 2010, 42(4): 425-432. |
| [15] | 杨祖耀, 詹思延, 王波, 吕晓珍, 舒正, 何英剑, 邱宁, 杨慧英. 中国血流感染住院病死率的系统评价和meta分析[J]. 北京大学学报(医学版), 2010, 42(3): 304-307. |
|
||