北京大学学报(医学版) ›› 2021, Vol. 53 ›› Issue (2): 240-245. doi: 10.19723/j.issn.1671-167X.2021.02.002
超细颗粒物对大鼠离体灌注心脏功能的影响
- 山西医科大学附属第一医院心内科,山西医科大学基础医学院药理教研室,太原 030001
Effects of ultrafine particulates on cardiac function in rat isolated heart
BAI Feng,HE Yi-fan,NIU Ya-nan,YANG Ruo-juan,CAO Jing( )
)
- Department of Cardiology, The First Hospital of Shanxi Medical University & Department of Pharmacology, Basic Medical School, Shanxi Medical University, Taiyuan 030001, China
摘要:
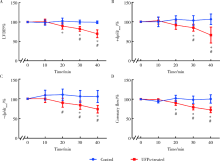
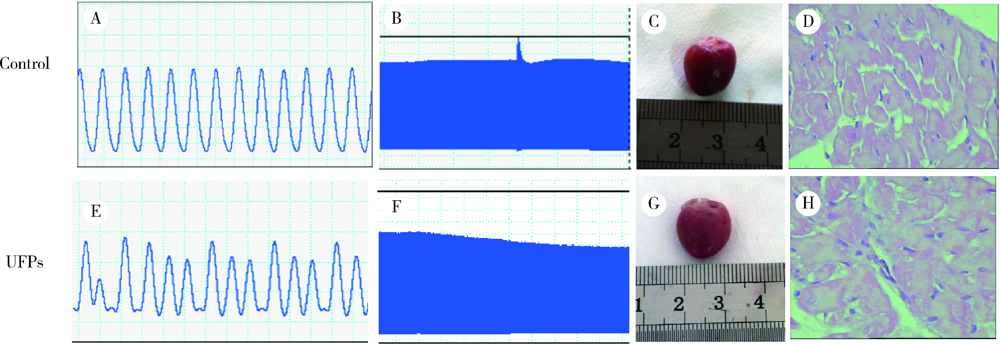
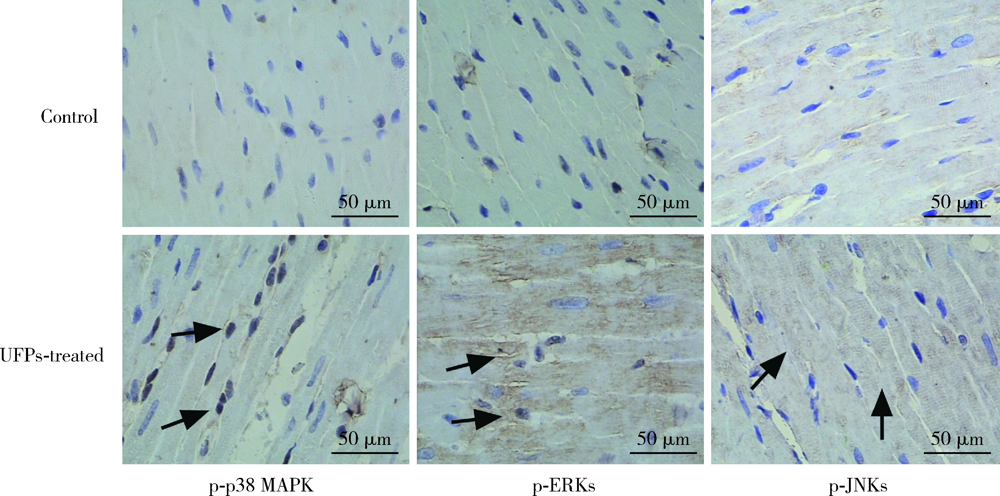
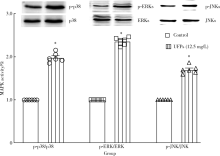
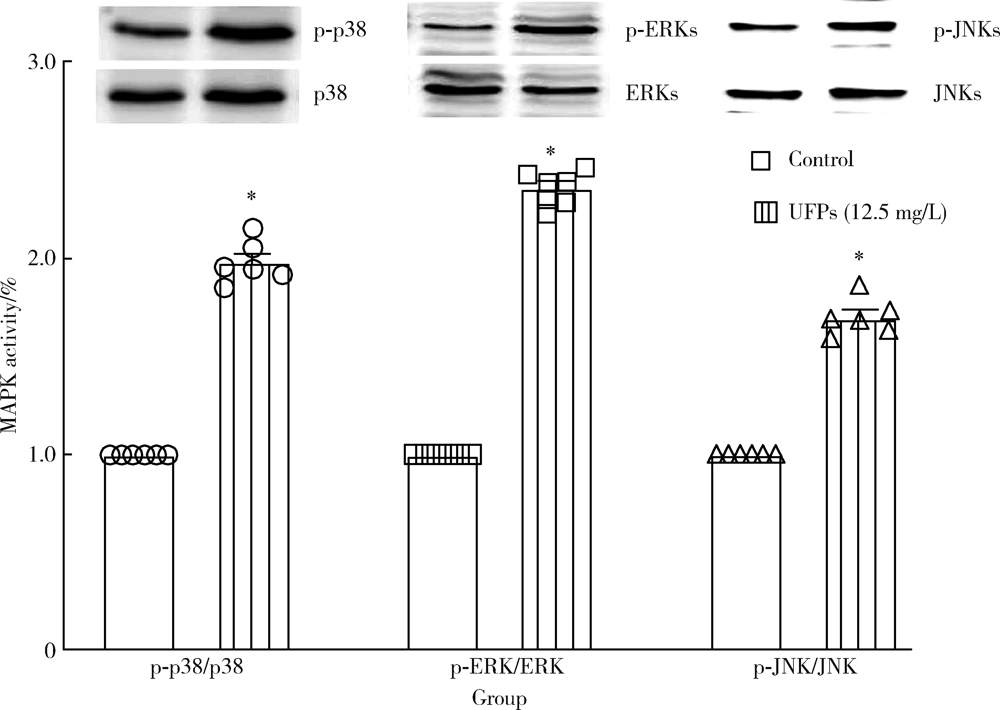
目的: 研究超细颗粒物(ultrafine particulates,UFPs)对大鼠离体心脏功能的影响及其机制。方法: 以含或不含UFPs的台式液经Langendorff系统持续灌流大鼠离体心脏模型40 min,观察灌流前后两组大鼠心脏血流动力学指标[左心室舒张压(left ventricular developed pressure,LVDP)、左心室内压最大上升和最大下降速率(±dp/dtmax)及冠脉流量(coronary flow,CF)]的变化。收集肺动脉流出液,采用硫代巴比妥酸法测定丙二醛(malondialdehyde,MDA),水溶性四唑盐法测定超氧化物歧化酶(superoxide dismutase,SOD), 比色法测定总抗氧化能力(total antioxidant capacity,TAOC)。免疫组织化学法和Western blots法测定两组心脏标本p-p38 MAPK、p-JNKs、p-ERKs的表达。结果: 相对于对照组,UFPs灌流组大鼠离体心脏功能指标LVDP、+dp/dtmax、-dp/dtmax、CF分别从(82.6 ± 2.1) mmHg、(1 624 ± 113) mmHg/s、(1 565 ± 116) mmHg/s、(12.0 ± 0.2) mL/min降至灌注结束时的(56.8 ± 4.4) mmHg、(1 066 ± 177) mmHg/s、(1 082 ± 134) mmHg/s、(8.7 ± 0.3) mL/min,各指标灌流结束时相比灌流初始值差异均有统计学意义(P<0.05)。UFPs灌流组肺动脉流出液MDA含量明显高于对照组[(1.95±0.18) nmol/L vs. (0.98±0.14) nmol/L,P<0.05],而SOD、TAOC明显低于对照组[(6.50±1.04) U/mL vs. (12.50±1.87) U/mL,(3.67±0.82) U/mL vs. (6.83±1.16) U/mL, P<0.05 ]。UFPs灌流组p-p38 MAPK、p-JNKs、p-ERKs 较对照组表达明显增加(P<0.05)。结论: UFPs短期暴露对大鼠离体心脏有直接的急性毒性作用,其作用机制可能与氧化应激及MAPK信号通路激活有关。
中图分类号:
- R122.7
| [1] | Klompmaker JO, Montagne DR, Meliefste K, et al. Spatial variation of ultrafine particles and black carbon in two cities: results from a short-term measurement campaign[J]. Sci Total Environ, 2015,508:266-275. |
| [2] |
Zareba W, Couderc JP, Oberdörster G, et al. ECG parameters and exposure to carbon ultrafine particles in young healthy subjects[J]. Inhal Toxicol, 2009,21(3):223-233.
doi: 10.1080/08958370802492407 pmid: 18991063 |
| [3] |
Laumbach RJ, Kipen HM, Ko S, et al. A controlled trial of acute effects of human exposure to traffic particles on pulmonary oxidative stress and heart rate variability[J]. Part Fibre Toxicol, 2014,11:45.
pmid: 25361615 |
| [4] |
Mills NL, Amin N, Robinson SD, et al. Do inhaled carbon nanoparticles translocate directly into the circulation in humans[J]. Am J Respir Crit Care Med, 2006,173(4):426-431.
pmid: 16339922 |
| [5] |
Lundbäck M, Mills NL, Lucking A, et al. Experimental exposure to diesel exhaust increases arterial stiffness in man[J]. Part Fibre Toxicol, 2009,6:7.
pmid: 19284640 |
| [6] |
Wang M, Beelen R, Stafoggia M, et al. Long-term exposure to elemental constituents of particulate matter and cardiovascular mortality in 19 European cohorts: results from the ESCAPE and TRANSPHORM projects[J]. Environ Int, 2014,66:97-106.
doi: 10.1016/j.envint.2014.01.026 pmid: 24561271 |
| [7] |
Stewart JC, Chalupa DC, Devlin RB, et al. Vascular effects of ultrafine particles in persons with type 2 diabetes[J]. Environ Health Perspect, 2010,118(12):1692-1698.
pmid: 20822968 |
| [8] |
Nemmar A, Subramaniyan D, Yasin J, et al. Impact of experimental type 1 diabetes mellitus on systemic and coagulation vulnerability in mice acutely exposed to diesel exhaust particles[J]. Part Fibre Toxicol, 2013,10:14.
pmid: 23587270 |
| [9] |
Rückerl R, Phipps RP, Schneider A, et al. Ultrafine particles and platelet activation in patients with coronary heart disease: results from a prospective panel study[J]. Part Fibre Toxicol, 2007,4:1.
doi: 10.1186/1743-8977-4-1 pmid: 17241467 |
| [10] |
Sun Q, Yue P, Ying Z, et al. Air pollution exposure potentiates hypertension through reactive oxygen species-mediated activation of Rho/ROCK[J]. Arterioscler Thromb Vasc Biol, 2008,28(10):1760-1766.
pmid: 18599801 |
| [11] | Simkhovich BZ, Marjoram P, Kleinman MT, et al. Direct and acute cardiotoxicity of ultrafine particles in young adult and old rat hearts[J]. Basic Res Cardiol, 2007,102(6):467-475. |
| [12] | Shaw CA, Robertson S, Miller MR, et al. Diesel exhaust particulate: exposed macrophages cause marked endothelial cell activation[J]. Am J Respir Cell Mol Biol, 2011,44(6):840-851. |
| [13] | Kim JB, Kim C, Choi E, et al. Particulate air pollution induces arrhythmia via oxidative stress and calcium calmodulin kinase Ⅱ activation[J]. Toxicol Appl Pharmacol, 2012,259(1):66-73. |
| [14] |
Cozzi E, Hazarika S, Stallings HW, et al. Ultrafine particulate matter exposure augments ischemia-reperfusion injury in mice[J]. Am J Physiol Heart Circ Physiol, 2006,291(2):H894-903.
pmid: 16582015 |
| [15] | Cao J, Qin G, Shi RZ, et al. Overproduction of reactive oxygen species and activation of MAPKs are involved in apoptosis induced by PM2.5 in rat cardiac H9C2 cells[J]. J Apple Tocicol, 2016,36(4):609-617. |
| [16] |
Baines CP, Molkentin JD. STRESS signaling pathways that modulate cardiac myocyte apoptosis[J]. J Mol Cell Cardiol, 2005,38(1):47-62.
doi: 10.1016/j.yjmcc.2004.11.004 pmid: 15623421 |
| [17] |
Zhu W, Zou Y, Aikawa R, et al. MAPK superfamily plays an important role in daunomycin-induced apoptosis of cardiac myocytes[J]. Circulation, 1999,100(20):2100-2107.
pmid: 10562267 |
| [18] |
Jarvis IW, Bergvall C, Morales DA, et al. Nanomolar levels of PAHs in extracts from urban air induce MAPK signaling in HepG2 cells[J]. Toxicol Lett, 2014,229(1):25-32.
pmid: 24910982 |
| [19] |
Rui W, Guan L, Zhang F, et al. PM2.5-induced oxidative stress increases adhesion molecules expression in human endothelial cells through the ERK/AKT/NF-κB-dependent pathway[J]. J Appl Toxicol, 2016,36(1):48-59.
pmid: 25876056 |
| [1] | 刘志伟,刘鹏,孟凡星,李天水,王颖,高嘉琪,周佐邑,王聪,赵斌. 内源性二氧化硫对脓毒症大鼠心肌氧化应激的调节[J]. 北京大学学报(医学版), 2023, 55(4): 582-586. |
| [2] | 轩艳,蔡宇,王啸轩,石巧,邱立新,栾庆先. 牙龈卟啉单胞菌感染对载脂蛋白e基因敲除小鼠动脉粥样硬化的影响[J]. 北京大学学报(医学版), 2020, 52(4): 743-749. |
| [3] | 王昊,陈亮,叶小云. 雷公藤甲素对TM4细胞氧化应激及PI3K/AKT通路的影响[J]. 北京大学学报(医学版), 2018, 50(4): 607-612. |
| [4] | 张怡,宋晓明,赵茜,王童,李丽娟,陈婕,徐洪兵,刘贝贝,孙晓燕,贺蓓,黄薇. 大气颗粒物及多环芳烃暴露与慢性阻塞性肺疾病患者全身性氧化应激水平[J]. 北京大学学报(医学版), 2017, 49(3): 394-402. |
| [5] | 和璐. 牙周炎和代谢综合征[J]. 北京大学学报(医学版), 2011, 43(1): 13-17. |
|
||