北京大学学报(医学版) ›› 2021, Vol. 53 ›› Issue (2): 246-254. doi: 10.19723/j.issn.1671-167X.2021.02.003
18F-FDG PET/CT半定量参数、表皮生长因子受体和间变淋巴瘤激酶基因突变对肺腺癌患者预后评估的价值
廖栩鹤1,王荣福1,Δ( ),刘萌1,Δ(
),刘萌1,Δ( ),陈雪祺1,熊焰2,农琳2,殷雷1,张炳晔1,杜毓菁1
),陈雪祺1,熊焰2,农琳2,殷雷1,张炳晔1,杜毓菁1
- 1.核医学科, 北京大学第一医院 北京 100034
2.病理科, 北京大学第一医院 北京 100034
Semiquantitative parameters of 18F-FDG PET/CT, gene mutation states of epidermal growth factor receptor and anaplastic lymphoma kinase in prognosis evaluation of patients with lung adenocarcinoma
LIAO Xu-he1,WANG Rong-fu1,Δ( ),LIU Meng1,Δ(
),LIU Meng1,Δ( ),CHEN Xue-qi1,XIONG Yan2,NONG Lin2,YIN Lei1,ZHANG Bing-ye1,DU Yu-jing1
),CHEN Xue-qi1,XIONG Yan2,NONG Lin2,YIN Lei1,ZHANG Bing-ye1,DU Yu-jing1
- 1. Department of Nuclear Medicine, Peking University First Hospital, Beijing 100034, China
2. Department of Pathology, Peking University First Hospital, Beijing 100034, China
摘要:
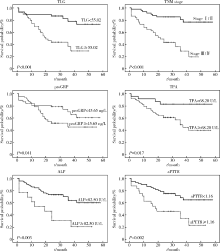
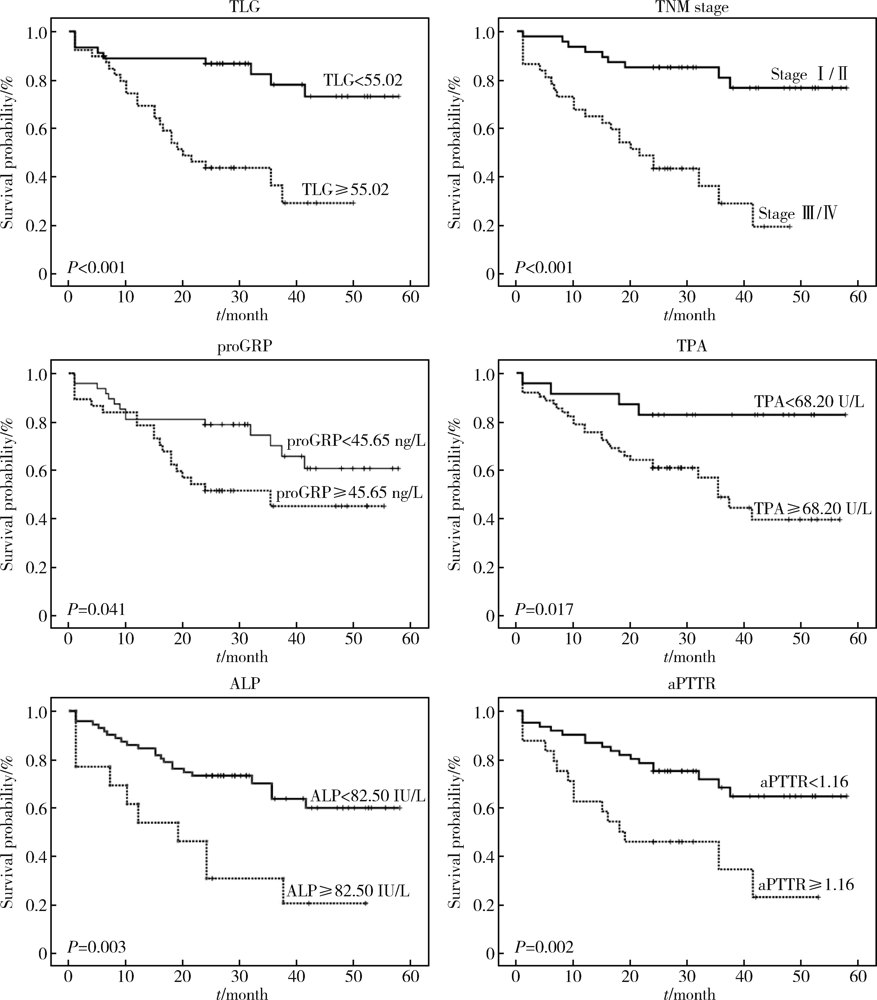
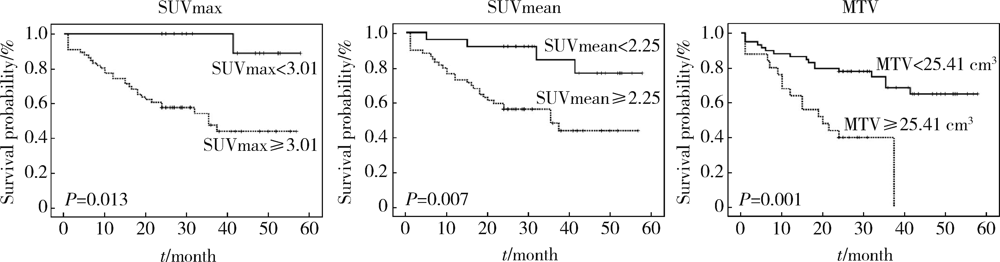
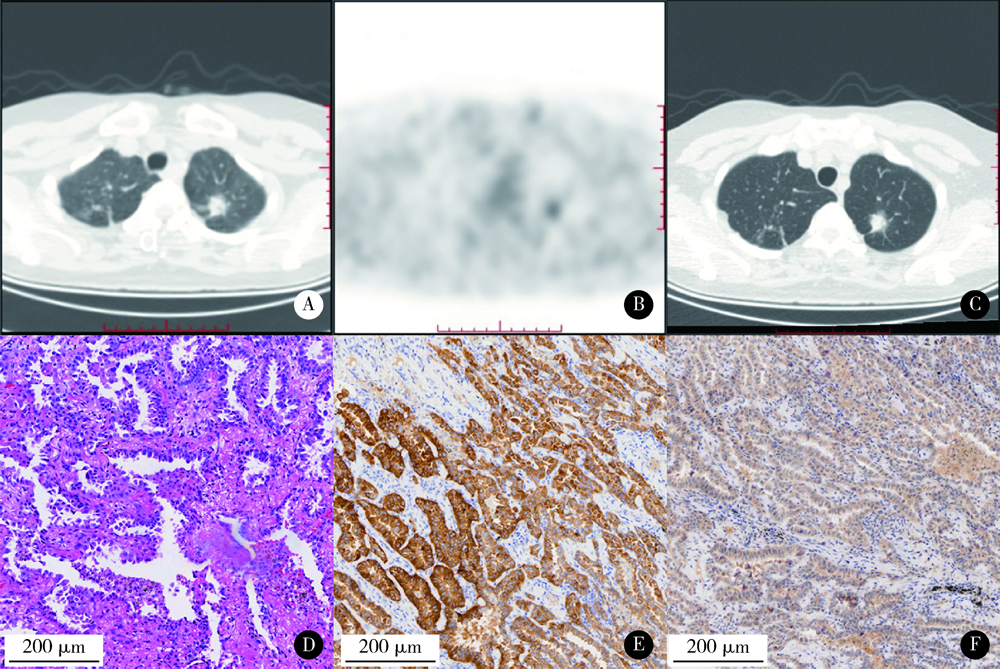
目的: 评估18F-FDG PET/CT半定量参数、表皮生长因子受体(epidermal growth factor receptor,EGFR)和间变淋巴瘤激酶(anaplastic lymphoma kinase,ALK)基因突变状态对肺腺癌患者预后评估的价值。方法: 回顾性收集84名肺腺癌患者术前18F-FDG PET/CT半定量参数,EGFR及ALK基因突变检查结果。18F-FDG PET/CT半定量参数分别为:最大标准化摄取值(maximum standardized uptake value,SUVmax)、平均标准化摄取值(average of standardized uptake value,SUVmean)、肿瘤代谢体积(metabolic tumor volume,MTV)和总糖酵解量(total lesion glycolysis,TLG)。连续变量用ROC曲线分析法转为分类变量,生存分析采用Cox比例风险回归分析,生存曲线经Log-rank检验和Kaplan-Meier法获得。结果: 患者平均随访期31个月(24~58个月)。单因素分析显示,原发灶SUVmax、SUVmean、MTV及TLG与无进展生存期(progression-free survival,PFS)显著相关。多因素Cox比例风险回归分析显示,不论年龄、性别、合并症、EGFR或ALK基因突变与否及治疗情况,TLG(≥55.02,HR=4.965,95%CI:1.360~18.133)、TNM分期(Ⅲ/Ⅳ期,HR=7.811,95%CI:2.977~20.489)、胃泌素释放肽前体(pro-gastrin releasing peptide,proGRP)(≥45.65 ng/L,HR=4.070,95%CI:1.442~11.487)、组织多肽抗原(tissue polypeptide antigen,TPA)(≥68.20 U/L,HR=6.996,95%CI:1.458~33.574)、碱性磷酸酶(alkaline phosphatase,ALP)(≥82.50 IU/L,HR=4.160,95%CI:1.416~12.219)和活化部分凝血活酶时间比值(ratio of activated partial thromboplastin time,aPTTR)(≥1.16,HR=4.576,95%CI:1.913~10.946)为独立显著预后因素。EGFR(P=0.343)或ALK(P=0.608)基因突变状态均与PFS无显著相关。结论: 原发灶高水平18F-FDG PET/CT半定量参数(SUVmax、SUVmean、MTV和TLG)对肺腺癌患者具有不同程度的预后评估价值,TNM分期、proGRP、TPA、ALP和aPTTR均与PFS存在独立、显著关联,EGFR或ALK基因突变状态与PFS未见明确相关。
中图分类号:
- R734.2
| [1] | Noone A, Howlader N, Krapcho M, et al. SEER cancer statistics review (CRS), 1975-2015[EB/OL]. (2018-09-10) [2018-09-10]. https://seer.cancer.gov/csr/1975_2015/. |
| [2] |
Brundage MD, Davies D, Mackillop WJ. Prognostic factors in non-small cell lung cancer: a decade of progress[J]. Chest, 2002,122(3):1037-1057.
doi: 10.1378/chest.122.3.1037 pmid: 12226051 |
| [3] |
Sharma A, Mohan A, Bhalla AS, et al. Role of various metabolic parameters derived from baseline 18F-FDG PET/CT as prognostic markers in non-small cell lung cancer patients undergoing platinum-based chemotherapy[J]. Clin Nucl Med, 2018,43(1):e8-e17.
pmid: 29112011 |
| [4] |
Salavati A, Duan F, Snyder BS, et al. Optimal FDG PET/CT volumetricparameters for risk stratification in patients with locally advanced non-small cell lung cancer: results from the ACRIN 6668/RTOG 0235 trial[J]. Eur J Nucl Med Mol Imaging, 2017,44(12):1969-1983.
doi: 10.1007/s00259-017-3753-x pmid: 28689281 |
| [5] |
Wang WT, Li Y, Ma J, et al. Serum carcinoembryonic antigen levels before initial treatment are associated with EGFR mutations and EML4-ALK fusion gene in lung adenocarcinoma patients[J]. Asian Pac J Cancer Prev, 2014,15(9):3927-3932.
doi: 10.7314/apjcp.2014.15.9.3927 pmid: 24935562 |
| [6] |
Chung HW, Lee KY, Kim HJ, et al. FDG PET/CT metabolic tumor volume and total lesion glycolysis predict prognosis in patients with advanced lung adenocarcinoma[J]. J Cancer Res Clin Oncol, 2014,140(1):89-98.
doi: 10.1007/s00432-013-1545-7 pmid: 24194352 |
| [7] |
Nisman B, Amir G, Lafair J, et al. Prognostic value of CYFRA 21-1, TPS and CEA in different histologic types of non-small cell lung cancer[J]. Anticancer Res, 1999,19(4C):3549-3552.
pmid: 10629651 |
| [8] |
Ma W, Wang M, Li X, et al. Quantitative 18F-FDG PET analysis in survival rate prediction of patients with non-small cell lung cancer[J]. Oncol Lett, 2018,16(4):4129-4136.
doi: 10.3892/ol.2018.9166 pmid: 30214552 |
| [9] |
Melloni G, Gajate AM, Sestini S, et al. New positron emission tomography derived parameters as predictive factors for recurrence in resected stage Ⅰ non-small cell lung cancer[J]. Eur J Surg Oncol, 2013,39(11):1254-1261.
doi: 10.1016/j.ejso.2013.07.092 pmid: 23948705 |
| [10] |
Hyun SH, Choi JY, Kim K, et al. Volume-based parameters of 18F-fluorodeoxyglucose positron emission tomography/computed tomography improve outcome prediction in early-stage non-small cell lung cancer after surgical resection[J]. Ann Surg, 2013,257(2):364-370.
pmid: 22968069 |
| [11] |
Kurtipek E, Cayci M, Duzgun N, et al. 18F-FDG PET/CT mean SUV and metabolic tumor volume for mean survival time in non-small cell lung cancer[J]. Clin Nucl Med, 2015,40(6):459-463.
doi: 10.1097/RLU.0000000000000740 pmid: 25742234 |
| [12] |
Camidge DR, Kono SA, Lu X, et al. Anaplastic lymphoma kinase gene rearrangements in non-small cell lung cancer are associated with prolonged progression-free survival on pemetrexed[J]. J Thorac Oncol, 2011,6(4):774-780.
pmid: 21336183 |
| [13] |
Izar B, Sequist L, Lee M, et al. The impact of EGFR mutation status on outcomes in patients with resected stage Ⅰ non-small cell lung cancers[J]. Ann Thorac Surg, 2013,96(3):962-968.
pmid: 23932319 |
| [14] |
Nisman B, Biran H, Ramu N, et al. The diagnostic and prognostic value of ProGRP in lung cancer[J]. Anticancer Res, 2009,29(11):4827-4832.
pmid: 20032442 |
| [15] |
Cistaro A, Quartuccio N, Mojtahedi A, et al. Prediction of 2 years-survival in patients with stage Ⅰ and Ⅱ non-small cell lung cancer utilizing (18)F-FDG PET/CT SUV quantification[J]. Radiol Oncol, 2013,47(3):219-223.
pmid: 24133385 |
| [16] |
Soussan M, Chouahnia K, Maisonobe JA, et al. Prognostic implications of volume-based measurements on FDG PET/CT in stage Ⅲ non-small-cell lung cancer after induction chemotherapy[J]. Eur J Nucl Med Mol Imaging, 2013,40(5):668-676.
pmid: 23306807 |
| [17] | Liu J, Dong M, Sun X, et al. Prognosticvalue of 18F-FDG PET/CT in surgical non-small cell lung cancer: a meta-analysis[J]. PLoS One, 2016,11(1):e146195. |
| [18] |
Ohtaka K, Hida Y, Kaga K, et al. Outcome analysis of 18F-fluorodeoxyglucose positron-emission tomography in patients with lung cancer after partial volume correction[J]. Anticancer Res, 2013,33(11):5193-5198.
pmid: 24222169 |
| [19] |
Caicedo C, Garcia-Velloso MJ, Lozano MD, et al. Role of 18F-FDG PET in prediction of KRAS and EGFR mutation status in patients with advanced non-small-cell lung cancer[J]. Eur J Nucl Med Mol Imaging, 2014,41(11):2058-2065.
pmid: 24990403 |
| [20] |
Liu A, Han A, Zhu H, et al. The role of metabolic tumor volume (MTV) measured by [18F] FDG PET/CT in predicting EGFR gene mutation status in non-small cell lung cancer[J]. Oncotarget, 2017,8(20):33736-33744.
pmid: 28422710 |
| [21] | Li Q, Zhang J, Cheng W, et al. Prognostic value of maximum standard uptake value, metabolic tumor volume, and total lesion glycolysis of positron emission tomography/computed tomography in patients with nasopharyngeal carcinoma: A systematic review and meta-analysis[J]. Medicine (Baltimore), 2017,96(37):e8084. |
| [22] |
Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma[J]. N Engl J Med, 2009,361(10):947-957.
pmid: 19692680 |
| [23] |
Koizumi T, Fukushima T, Gomi D, et al. Correlation of early PET findings with tumor response to molecular targeted agents in patients with advanced driver-mutated non-small cell lung cancer[J]. Med Oncol, 2017,34(10):169.
doi: 10.1007/s12032-017-1032-0 pmid: 28864950 |
| [24] |
Foa P, Fornier M, Miceli R, et al. Tumour markers CEA, NSE, SCC, TPA and CYFRA 21.1 in resectable non-small cell lung cancer[J]. Anticancer Res, 1999,19(4C):3613-3618.
pmid: 10629660 |
| [25] |
Alatas F, Alatas O, Metintas M, et al. Usefulness of bone mar-kers for detection of bone metastases in lung cancer patients[J]. Clin Biochem, 2002,35(4):293-296.
pmid: 12135691 |
| [26] |
Qi Y, Fu J. Research on the coagulation function changes in non small cell lung cancer patients and analysis of their correlation with metastasis and survival[J]. J BUON, 2017,22(2):462-467.
pmid: 28534370 |
| [27] | Ettinger DS, Wood DE, Aisner DL, et al. National Comprehensive Cancer Network (NCCN). NCCN clinical practice guidelines in oncology: non-small cell lung cancer (2019V2)[EB/OL]. (2018-11-21) [2018-11-21]. https://www.nccn.org/professionals/physician_gls/default.aspx. |
| [1] | 黄教悌,胡菁,韩博. 治疗相关神经内分泌前列腺癌机制研究与靶向治疗新进展[J]. 北京大学学报(医学版), 2024, 56(4): 557-561. |
| [2] | 邢念增,王明帅,杨飞亚,尹路,韩苏军. 前列腺免活检创新理念的临床实践及其应用前景[J]. 北京大学学报(医学版), 2024, 56(4): 565-566. |
| [3] | 颜野,李小龙,夏海缀,朱学华,张羽婷,张帆,刘可,刘承,马潞林. 前列腺癌根治术后远期膀胱过度活动症的危险因素[J]. 北京大学学报(医学版), 2024, 56(4): 589-593. |
| [4] | 于书慧,韩佳凝,钟丽君,陈聪语,肖云翔,黄燕波,杨洋,车新艳. 术前盆底肌电生理参数对前列腺癌根治性切除术后早期尿失禁的预测价值[J]. 北京大学学报(医学版), 2024, 56(4): 594-599. |
| [5] | 朱晓娟,张虹,张爽,李东,李鑫,徐玲,李挺. 人表皮生长因子受体2低表达乳腺癌的临床病理学特征及预后[J]. 北京大学学报(医学版), 2023, 55(2): 243-253. |
| [6] | 沈棋,刘亿骁,何群. 肾黏液样小管状和梭形细胞癌的临床病理特点及预后[J]. 北京大学学报(医学版), 2023, 55(2): 276-282. |
| [7] | 宁博涵,张青霞,杨慧,董颖. 伴间质细胞增生、玻璃样变性及索状结构的子宫内膜样腺癌1例[J]. 北京大学学报(医学版), 2023, 55(2): 366-369. |
| [8] | 曹芳,钟明,刘从容. 宫体POLE突变型内膜样癌合并HPV感染相关性宫颈腺癌1例报道及文献回顾[J]. 北京大学学报(医学版), 2023, 55(2): 370-374. |
| [9] | 方伟岗,田新霞,解云涛. 基因多态性对中国汉族女性乳腺癌遗传易感性的影响[J]. 北京大学学报(医学版), 2022, 54(5): 822-828. |
| [10] | 王跃,张爽,张虹,梁丽,徐玲,程元甲,段学宁,刘荫华,李挺. 激素受体阳性/人表皮生长因子受体2阴性乳腺癌临床病理特征及预后[J]. 北京大学学报(医学版), 2022, 54(5): 853-862. |
| [11] | 刘圣杰,侯惠民,吕政通,丁鑫,王璐,张磊,刘明. 双极雄激素序贯免疫检查点抑制剂治疗转移性去势抵抗性前列腺癌4例[J]. 北京大学学报(医学版), 2022, 54(4): 766-769. |
| [12] | 顾阳春,刘颖,谢超,曹宝山. 程序性死亡蛋白-1抑制剂治疗晚期肺癌出现垂体免疫不良反应3例[J]. 北京大学学报(医学版), 2022, 54(2): 369-375. |
| [13] | 白杲琛,宋毅,金杰,虞巍,何志嵩. 多西他赛联合卡铂治疗转移性去势抵抗性前列腺癌的临床疗效[J]. 北京大学学报(医学版), 2021, 53(4): 686-691. |
| [14] | 王迎春,黄永辉,常虹,姚炜,闫秀娥,李柯,张耀鹏,郑炜. 十二指肠乳头息肉良、恶性病变比较及活检准确性[J]. 北京大学学报(医学版), 2021, 53(1): 204-209. |
| [15] | 鲍轶,莫娟芬. 同时性多原发肺腺癌组织编码转录因子ERG基因相同位点突变1例报告[J]. 北京大学学报(医学版), 2020, 52(5): 971-974. |
|
||