北京大学学报(医学版) ›› 2022, Vol. 54 ›› Issue (2): 369-375. doi: 10.19723/j.issn.1671-167X.2022.02.027
程序性死亡蛋白-1抑制剂治疗晚期肺癌出现垂体免疫不良反应3例
- 1.北京大学第三医院 肿瘤化疗与放射病科,北京 100191
2.北京大学第三医院 放射科,北京 100191
3.北京大学第三医院 内分泌科,北京 100191
Pituitary immune-related adverse events induced by programmed cell death protein 1 inhibitors in advanced lung cancer patients: A report of 3 cases
GU Yang-chun1,LIU Ying2,XIE Chao3,CAO Bao-shan1,△( )
)
- 1. Department of Medical Oncology and Radiation Sickness, Peking University Third Hospital, Beijing 100191, China
2. Department of Radiology, Peking University Third Hospital, Beijing 100191, China
3. Department of Endocrinology, Peking University Third Hospital, Beijing 100191, China
摘要:
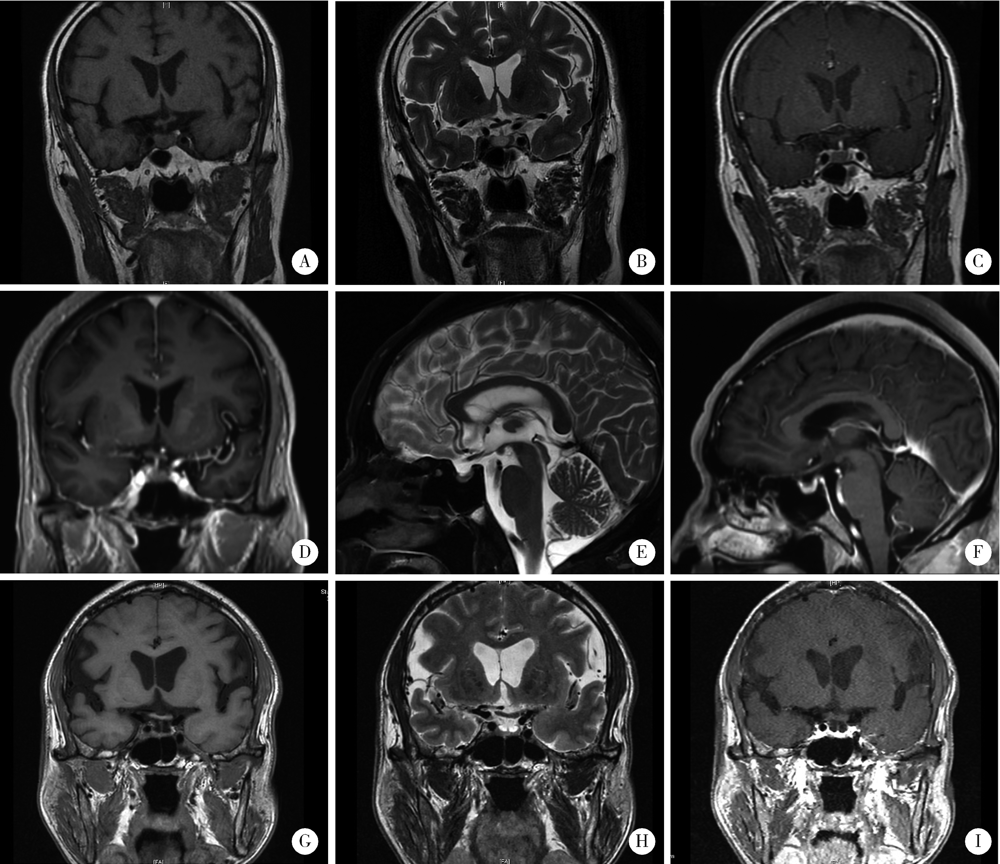
程序性死亡蛋白-1(programmed cell death protein 1,PD-1)及其配体-1(PD-1 ligand 1,PD-L1)的抑制剂广泛用于肺癌治疗,但引起的免疫相关不良反应(immune related adverse events,irAEs)值得关注。垂体irAEs包括垂体炎和垂体功能减退,常见于细胞毒T淋巴细胞相关抗原-4抑制剂治疗后,而较少见于PD-1/PD-L1抑制剂治疗后。孤立性促肾上腺皮质激素(adrenocorticotropic hormone,ACTH)缺乏是垂体irAEs的一种特殊亚型,不伴垂体其他功能紊乱和垂体肿大。本研究报告3例晚期肺癌患者,PD-1抑制剂治疗后出现孤立性ACTH缺乏及其他irAEs。病例1是68岁男性患者,先确诊PD-L1高表达的肺腺癌,采用帕博利珠单抗(pembrolizumab)单药治疗,期间出现免疫性肝炎,经高剂量甲基泼尼松龙[0.5~1.0 mg/(kg·d)]治疗后缓解;间隔11个月又确诊原发性胃癌,故在帕博利珠单抗基础上增加阿帕替尼(apatinib)治疗;帕博利珠单抗治疗共17次后,患者的肺癌和胃癌均未进展,但出现严重恶心和无力,此时甲基泼尼松龙已停药10个月,血液生化检查提示重度低钠血症(121 mmol/L,参考值137~147 mmol/L,下同),8:00 a.m.皮质醇(<1 μg/dL,参考值5~25 μg/dL,下同)和ACTH(2.2 ng/L,参考值7.2~63.3 ng/L,下同)降低,但甲状腺功能、性激素和泌乳素均正常。病例2是66岁男性肺腺癌患者,参加新型PD-1抑制剂HX008联合化疗的Ⅱ期临床研究(登记号:CTR20202387)。治疗5个月(共7次用药)后,患者的肺癌达到部分缓解,但恶心和呕吐却突然加重,伴轻度呼吸困难和双下肢无力,其血液生化检查提示轻度低钠血症(135 mmol/L),8:00 a.m.皮质醇(4.3 μg/dL)和ACTH(1.5 ng/L)降低,但甲状腺功能正常;同时肺CT显示中度免疫性肺炎。病例3是63岁男性肺鳞状细胞癌患者,一线使用信迪利单抗(sintilimab)联合化疗,肺癌最佳疗效为部分缓解,仅出现轻度免疫性皮疹;治疗5周期后,肺癌进展,此后6个月未使用免疫治疗;再次免疫治疗前,常规评估发现8:00 a.m.血皮质醇降低(1.5 μg/dL),ACTH正常(8.0 ng/L),但无肾上腺皮质功能减退症状,使用替雷利珠单抗(tislelizumab)联合化疗2周期后出现肺部感染伴持续低热、中度无力和重度低钠血症(116 mmol/L),此时, 8:00 a.m.血皮质醇为3.1 μg/dL,ACTH为7.2 ng/L,甲状腺功能、性激素和泌乳素均正常。这3例患者均无头痛和视力障碍,脑磁共振成像均未见垂体肿大或垂体柄增粗,且无动态变化。患者均接受了泼尼松(2.5~5 mg/d)激素替代治疗,相关症状缓解后均恢复PD-1抑制剂治疗。病例2较特殊,其因同时伴有中度免疫性肺炎而采用高剂量泼尼松[1 mg/(kg·d)]治疗,并逐渐减量至生理替代剂量,8:00 a.m.血皮质醇和ACTH恢复并维持正常,但其他两例患者的垂体功能减退均未恢复。本组病例提示,PD-1抑制剂诱发的垂体irAEs可表现为孤立性ACTH缺乏,其发病时间跨度大,临床表现不特异,恢复模式也不同。因此,对PD-1抑制剂治疗的患者,尤其是疗效好的患者,要定期监测垂体相关内分泌激素水平,警惕垂体irAEs。
中图分类号:
- R730.51
| [1] |
Deligiorgi MV, Liapi C, Trafalis DT. Hypophysitis related to immune checkpoint inhibitors: An intriguing adverse event with many faces[J]. Expert Opin Biol Ther, 2021, 21(8):1097-1120.
doi: 10.1080/14712598.2021.1869211 |
| [2] |
Stelmachowska-Banaś M, Czajka-Oraniec I. Management of endocrine immune-related adverse events of immune checkpoint inhibitors: An updated review[J]. Endocr Connect, 2020, 9(10):R207-R228.
doi: 10.1530/EC-20-0342 pmid: 33064663 |
| [3] |
Barroso-Sousa R, Barry WT, Garrido-Castro AC, et al. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: A systematic review and meta-analysis[J]. JAMA Oncol, 2018, 4(2):173-182.
doi: 10.1001/jamaoncol.2017.3064 pmid: 28973656 |
| [4] |
de Filette J, Andreescu CE, Cools F, et al. A systematic review and meta-analysis of endocrine-related adverse events associated with immune checkpoint inhibitors[J]. Horm Metab Res, 2019, 51(3):145-156.
doi: 10.1055/a-0843-3366 pmid: 30861560 |
| [5] | Baxi S, Yang A, Gennarelli RL, et al. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: Systematic review and meta-analysis[J]. BMJ, 2018(360):k793. |
| [6] |
Kobayashi T, Iwama S, Yasuda Y, et al. Pituitary dysfunction induced by immune checkpoint inhibitors is associated with better overall survival in both malignant melanoma and non-small cell lung carcinoma: A prospective study[J]. J Immunother Cancer, 2020, 8(2):e000779.
doi: 10.1136/jitc-2020-000779 |
| [7] |
Di Dalmazi G, Ippolito S, Lupi I, et al. Hypophysitis induced by immune checkpoint inhibitors: A 10-year assessment[J]. Expert Rev Endocrinol Metab, 2019, 14(6):381-398.
doi: 10.1080/17446651.2019.1701434 pmid: 31842671 |
| [8] |
Takeno A, Yamamoto M, Morita M, et al. Late-onset isolated adrenocorticotropic hormone deficiency caused by nivolumab: A case report[J]. BMC Endocr Disord, 2019, 19(1):25.
doi: 10.1186/s12902-019-0335-x pmid: 30782163 |
| [9] |
Antoniou S, Bazazo G, Röckl L, et al. Late-onset hypophysitis after discontinuation of nivolumab treatment for advanced skin melanoma: A case report[J]. BMC Endocr Disord, 2021, 21(1):191.
doi: 10.1186/s12902-021-00854-y pmid: 34544399 |
| [10] |
Kanie K, Iguchi G, Bando H, et al. Two cases of atezolizumab-induced hypophysitis[J]. J Endocr Soc, 2017, 2(1):91-95.
doi: 10.1210/js.2017-00414 |
| [11] |
Ohara N, Kobayashi M, Ohashi K, et al. Isolated adrenocorticotropic hormone deficiency and thyroiditis associated with nivolumab therapy in a patient with advanced lung adenocarcinoma: A case report and review of the literature[J]. J Med Case Rep, 2019, 13(1):88.
doi: 10.1186/s13256-019-2002-2 |
| [12] |
Iwama S, Kobayashi T, Arima H. Clinical characteristics, ma-nagement, and potential biomarkers of endocrine dysfunction induced by immune checkpoint inhibitors[J]. Endocrinol Metab (Seoul), 2021, 36(2):312-321.
doi: 10.3803/EnM.2021.1007 |
| [13] | United States National Cancer Institute. Common terminology criteria for adverse events (CTCAE)[S/OL]. (2017-11-27). [2021-11-23]. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf. |
| [14] |
Nguyen H, Shah K, Waguespack SG, et al. Immune checkpoint inhibitor related hypophysitis: Diagnostic criteria and recovery patterns[J]. Endocr Relat Cancer, 2021, 28(7):419-431.
doi: 10.1530/ERC-20-0513 |
| [15] |
Faje A, Reynolds K, Zubiri L, et al. Hypophysitis secondary to nivolumab and pembrolizumab is a clinical entity distinct from ipilimumab-associated hypophysitis[J]. Eur J Endocrinol, 2019, 181(3):211-219.
doi: 10.1530/EJE-19-0238 |
| [16] |
Kanie K, Iguchi G, Bando H, et al. Mechanistic insights into immune checkpoint inhibitor-related hypophysitis: A form of paraneoplastic syndrome[J]. Cancer Immunol Immunother, 2021, 70(12):3669-3677.
doi: 10.1007/s00262-021-02955-y |
| [17] |
Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus chemo-therapy for squamous non-small-cell lung cancer[J]. N Engl J Med, 2018, 379(21):2040-2051.
doi: 10.1056/NEJMoa1810865 |
| [18] |
Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer[J]. N Engl J Med, 2018, 378(22):2078-2092.
doi: 10.1056/NEJMoa1801005 |
| [19] |
Amereller F, Deutschbein T, Joshi M, et al. Differences between immunotherapy-induced and primary hypophysitis: A multicenter retrospective study[J]. Pituitary, 2022, 25(1):152-158.
doi: 10.1007/s11102-021-01182-z |
| [20] |
Kurokawa R, Ota Y, Gonoi W, et al. MRI findings of immune checkpoint inhibitor-induced hypophysitis: Possible association with fibrosis[J]. Am J Neuroradiol, 2020; 41(9):1683-1689.
doi: 10.3174/ajnr.A6692 pmid: 32763900 |
| [21] |
Seethapathy H, Rusibamayila N, Chute DF, et al. Hyponatremia and other electrolyte abnormalities in patients receiving immune checkpoint inhibitors[J]. Nephrol Dial Transplant, 2021, 36(12):2241-2247.
doi: 10.1093/ndt/gfaa272 |
| [22] | 中华医学会内分泌学分会免疫内分泌学组. 免疫检查点抑制剂引起的内分泌系统免疫相关不良反应专家共识(2020)[J]. 中华内分泌代谢杂志, 2021, 37(1):1-16. |
| [23] | Management of Immunotherpy-Related Toxicities. National comphrehensive cancer network (NCCN) guidelines[R/OL]. Version 4. (2021-09-27) [2021-11-23]. https://www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf. |
| [24] | 中国临床肿瘤学会(CSCO). 免疫检查点抑制剂相关的毒性管理指南2021[R]. 北京: 人民卫生出版社, 2021. |
| [25] |
Cooksley T, Girotra M, Ginex P, et al. Multinational association of supportive care in cancer (MASCC) 2020 clinical practice re-commendations for the management of immune checkpoint inhibitor endocrinopathies and the role of advanced practice providers in the management of immune-mediated toxicities[J]. Support Care Cancer, 2020, 28(12):6175-6181.
doi: 10.1007/s00520-020-05709-1 |
| [26] |
Chang LS, Barroso-Sousa R, Tolaney SM, et al. Endocrine toxicity of cancer immunotherapy targeting immune checkpoints[J]. Endocr Rev, 2019, 40(1):17-65.
doi: 10.1210/er.2018-00006 |
| [27] | Faje AT, Lawrence D, Flaherty K, et al. High-dose glucocorticoids for the treatment of ipilimumab-induced hypophysitis is associated with reduced survival in patients with melanoma[J]. Can-cer, 2018, 124(18):3706-3714. |
| [28] |
Fernandes S, Varlamov EV, McCartney S, et al. A novel etiology of hypophysitis: Immune checkpoint inhibitors[J]. Endocrinol Metab Clin North Am, 2020, 49(3):387-399.
doi: 10.1016/j.ecl.2020.05.002 |
| [29] | Iwama S, De Remigis A, Callahan MK, et al. Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody[J]. Sci Transl Med, 2014, 6(230): 230ra45. |
| [30] |
Caturegli P, Di Dalmazi G, Lombardi M, et al. Hypophysitis se-condary to cytotoxic T-lymphocyte-associated protein 4 blockade: Insights into pathogenesis from an autopsy series[J]. Am J Pathol, 2016, 186(12):3225-3235.
doi: S0002-9440(16)30379-0 pmid: 27750046 |
| [31] |
Mihic-Probst D, Reinehr M, Dettwiler S, et al. The role of macrophages type 2 and T-regs in immune checkpoint inhibitor related adverse events[J]. Immunobiology, 2020, 225(5):152009.
doi: 10.1016/j.imbio.2020.152009 |
| [32] |
Kobayashi T, Iwama S, Sugiyama D, et al. Anti-pituitary antibo-dies and susceptible human leukocyte antigen alleles as predictive biomarkers for pituitary dysfunction induced by immune checkpoint inhibitors[J]. J Immunother Cancer, 2021, 9(5):e002493.
doi: 10.1136/jitc-2021-002493 |
| [1] | 刘家骏, 刘国康, 朱玉虎. 免疫相关性重症肺炎1例[J]. 北京大学学报(医学版), 2024, 56(5): 932-937. |
| [2] | 王薇,王佳宁,虞巍,朱赛楠,高莹,张俊清. 肾上腺性库欣综合征与无功能腺瘤患者的凝血功能比较及其影响因素[J]. 北京大学学报(医学版), 2023, 55(6): 1062-1067. |
| [3] | 应沂岑,唐琦,杨恺惟,米悦,范宇,虞巍,宋毅,何志嵩,周利群,李学松. 泌尿肿瘤免疫检查点抑制剂相关性肌炎的临床特征[J]. 北京大学学报(医学版), 2022, 54(4): 644-651. |
| [4] | 秦彩朋,宋宇轩,丁梦婷,王飞,林佳兴,杨文博,杜依青,李清,刘士军,徐涛. 肾癌免疫治疗疗效评估突变预测模型的建立[J]. 北京大学学报(医学版), 2022, 54(4): 663-668. |
| [5] | 刘圣杰,侯惠民,吕政通,丁鑫,王璐,张磊,刘明. 双极雄激素序贯免疫检查点抑制剂治疗转移性去势抵抗性前列腺癌4例[J]. 北京大学学报(医学版), 2022, 54(4): 766-769. |
| [6] | 廖栩鹤,王荣福,刘萌,陈雪祺,熊焰,农琳,殷雷,张炳晔,杜毓菁. 18F-FDG PET/CT半定量参数、表皮生长因子受体和间变淋巴瘤激酶基因突变对肺腺癌患者预后评估的价值[J]. 北京大学学报(医学版), 2021, 53(2): 246-254. |
| [7] | 鲍轶,莫娟芬. 同时性多原发肺腺癌组织编码转录因子ERG基因相同位点突变1例报告[J]. 北京大学学报(医学版), 2020, 52(5): 971-974. |
| [8] | 耿良,吕静,范敬. 肺瘤平膏联合环磷酰胺化疗对肺癌的抑瘤作用和酸性微环境的影响[J]. 北京大学学报(医学版), 2020, 52(2): 247-253. |
| [9] | 梅放,赵婷婷,高菲,郑杰. 肺罕见良性双相分化性肿瘤——肺腺纤维瘤1例并文献复习[J]. 北京大学学报(医学版), 2017, 49(6): 1076-1080. |
| [10] | 牟倩倩,余春华,李俊英. 肺癌初治患者心理痛苦的现状调查及相关因素分析[J]. 北京大学学报(医学版), 2016, 48(3): 507-514. |
| [11] | 倪莲芳, 刘新民. 血清肿瘤标记物对孤立性肺结节良恶性的诊断价值[J]. 北京大学学报(医学版), 2014, 46(5): 707-710. |
| [12] | 李彦,姜亮,刘晓光,刘忠军,韦峰,吴奉梁,党礌. 肺癌脊柱转移瘤的手术治疗疗效及生存分析[J]. 北京大学学报(医学版), 2014, 46(1): 138-143. |
| [13] | 吴齐, 曹长琦, 李士杰, 闫炎, 李香菊, 阎石, 吴楠, 张集昌. 超声内镜引导下经支气管针吸活检与经支气管针吸活检对肺与纵隔病变诊断价值的对比研究[J]. 北京大学学报(医学版), 2013, 45(03): 464-468. |
| [14] | Zhao-lin XU, Drew BETHUNE, Daria MANOS, Annette FOYLE, Harry . 肺玻璃样变肉芽肿病(英文稿)[J]. 北京大学学报(医学版), 2009, 41(4): 463-468. |
| [15] | 董爱梅, 尹洪芳, 高燕明, 郭晓蕙. 淋巴细胞性垂体炎患者自发再次妊娠1例[J]. 北京大学学报(医学版), 2009, 41(2): 242-244. |
| Viewed | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
Full text 301
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
Abstract 1139
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||
Cited |
|
|||||||||||||||||||||||||||||||||||||||||||||||||
| Shared | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Discussed | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
||