北京大学学报(医学版) ›› 2022, Vol. 54 ›› Issue (4): 663-668. doi: 10.19723/j.issn.1671-167X.2022.04.013
肾癌免疫治疗疗效评估突变预测模型的建立
秦彩朋,宋宇轩,丁梦婷,王飞,林佳兴,杨文博,杜依青,李清,刘士军,徐涛*( )
)
- 北京大学人民医院泌尿外科,北京 100044
Establishment of a mutation prediction model for evaluating the efficacy of immunotherapy in renal carcinoma
Cai-peng QIN,Yu-xuan SONG,Meng-ting DING,Fei WANG,Jia-xing LIN,Wen-bo YANG,Yi-qing DU,Qing LI,Shi-jun LIU,Tao XU*( )
)
- Department of Urology, Peking University People' s Hospital, Beijing 100044, China
摘要:
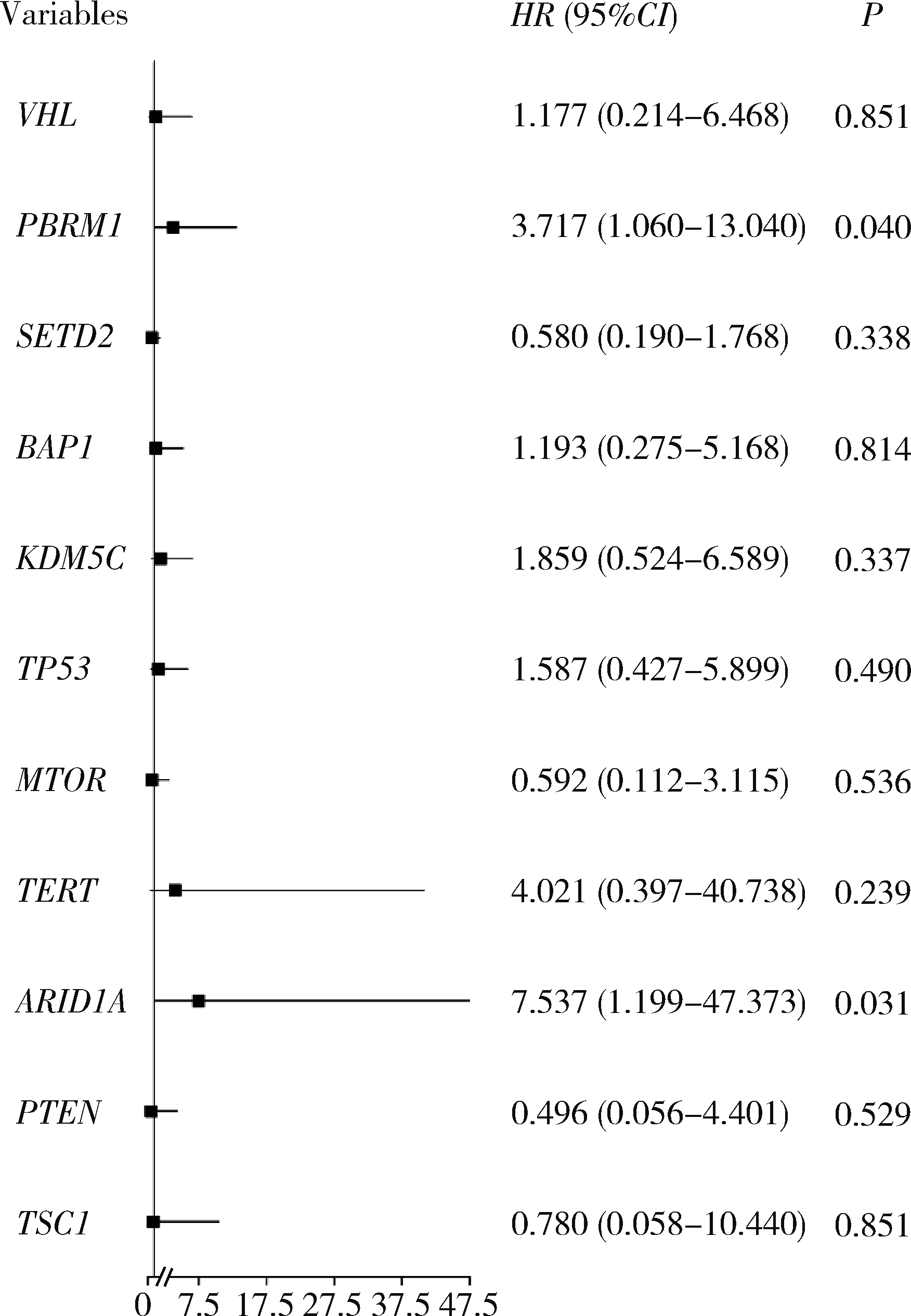
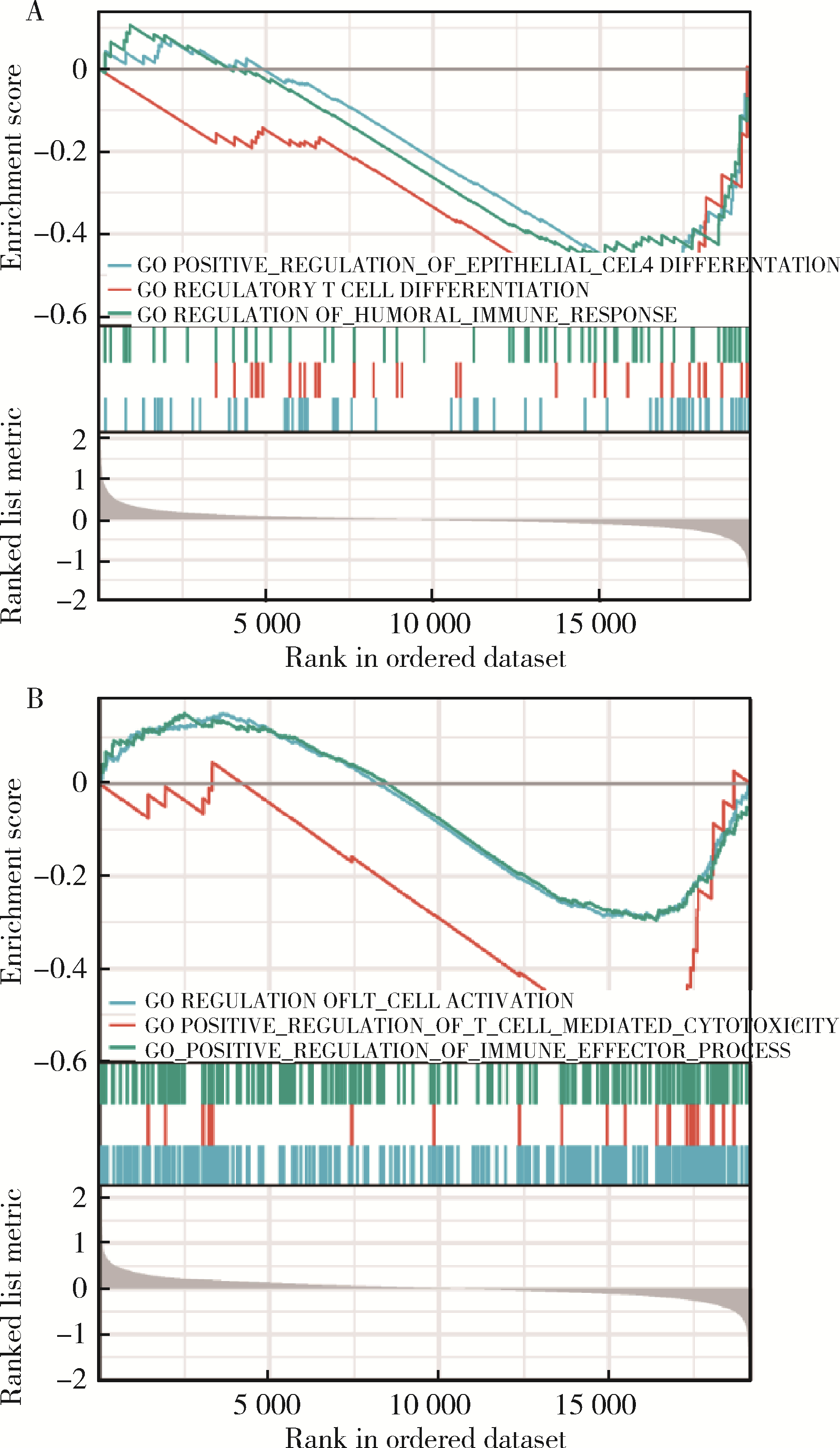
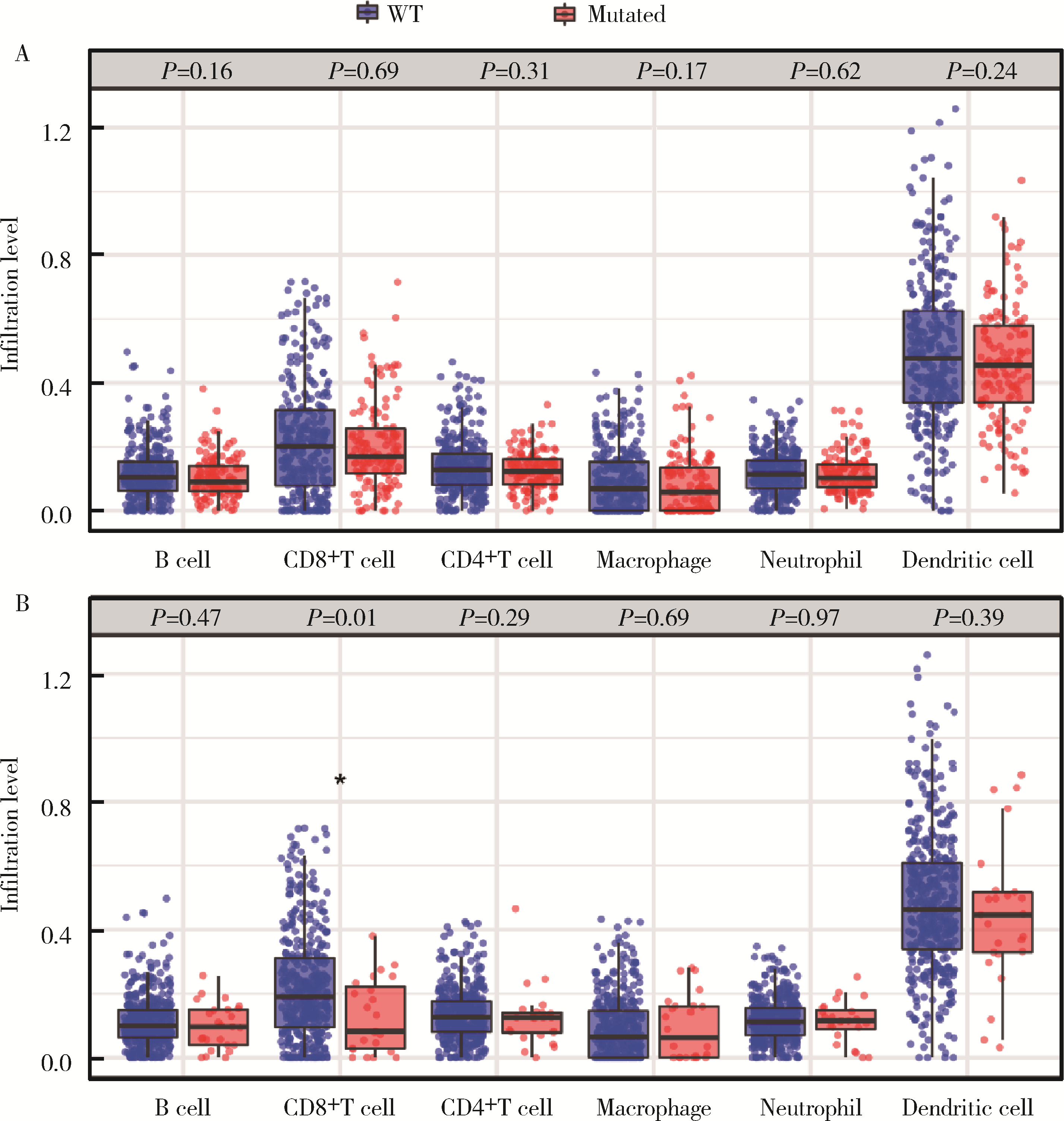
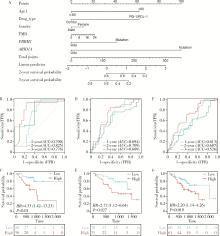
目的: 应用MSKCC(Memorial Sloan Kettering Cancer Center)泛癌免疫治疗队列肾癌患者的基因组测序数据建立疗效评估突变预测模型。方法: 针对MSKCC泛癌免疫治疗队列中121例接受免疫检查点抑制剂(immune checkpoint inhibitors, ICI)治疗的肾透明细胞癌患者基因组测序数据,应用Cox回归分析,鉴定与疗效相关的突变基因,并构建综合ICI药物疗效突变预测模型。结果: PBRM1和ARID1A突变与MSKCC泛癌免疫治疗队列肾癌患者治疗效果相关,基于此建立了年龄、性别、治疗类型、肿瘤突变负荷、PBRM1和ARID1A突变状态的疗效预测模型(1年生存AUC=0.700,2年生存AUC=0.825,3年生存AUC=0.776)。结论: PBRM1和ARID1A突变可作为肾癌免疫治疗疗效评估的潜在生物标志物,基于以上两基因突变状态建立的的疗效预测模型可用以筛选更适宜接受ICI免疫治疗的肾癌患者。
中图分类号:
- R737
| 1 |
Sung H , Ferlay J , Siegel RL , et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71 (3): 209- 249.
doi: 10.3322/caac.21660 |
| 2 |
Moch H , Cubilla AL , Humphrey PA , et al. The 2016 WHO classification of tumours of the urinary system and male genital organs: Part A: Renal, penile, and testicular tumours[J]. Eur Urol, 2016, 70 (1): 93- 105.
doi: 10.1016/j.eururo.2016.02.029 |
| 3 |
Ht C , McGovern F . Renal-cell carcinoma[J]. N Engl J Med, 2005, 353 (23): 2477- 2490.
doi: 10.1056/NEJMra043172 |
| 4 |
Bianchi M , Sun M , Jeldres C , et al. Distribution of metastatic sites in renal cell carcinoma: A population-based analysis[J]. Ann Oncol, 2012, 23 (4): 973- 980.
doi: 10.1093/annonc/mdr362 |
| 5 |
Everson TC , Cole WH . Spontaneous regression of cancer: Preliminary report[J]. Ann Surg, 1956, 144 (3): 366- 383.
doi: 10.1097/00000658-195609000-00007 |
| 6 | Janiszewska AD , Poletajew S , Wasiutyński A . Spontaneous regression of renal cell carcinoma[J]. Contemp Oncol (Pozn), 2013, 17 (2): 123- 127. |
| 7 |
Klapper JA , Downey SG , Smith FO , et al. High-dose interleukin-2 for the treatment of metastatic renal cell carcinoma: A retrospective analysis of response and survival in patients treated in the surgery branch at the National Cancer Institute between 1986 and 2006[J]. Cancer, 2008, 113 (2): 293- 301.
doi: 10.1002/cncr.23552 |
| 8 |
Motzer RJ , Escudier B , McDermott DF , et al. Nivolumab versus everolimus in advanced renal-cell carcinoma[J]. N Engl J Med, 2015, 373 (19): 1803- 1813.
doi: 10.1056/NEJMoa1510665 |
| 9 |
Motzer RJ , Tannir NM , McDermott DF , et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma[J]. N Engl J Med, 2018, 378 (14): 1277- 1290.
doi: 10.1056/NEJMoa1712126 |
| 10 | Plimack ER , Rini BI , Stus V , et al. Pembrolizumab plus axitinib versus sunitinib as first-line therapy for advanced renal cell carcinoma (RCC): Updated analysis of KEYNOTE-426[J]. J Clin Oncol, 2020, 38 (Suppl 15): 5001. |
| 11 |
Samstein RM , Lee CH , Shoushtari AN , et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types[J]. Nat Genet, 2019, 51 (2): 202- 206.
doi: 10.1038/s41588-018-0312-8 |
| 12 |
Braun DA , Hou Y , Bakouny Z , et al. Interplay of somatic alterations and immune infiltration modulates response to PD-1 blockade in advanced clear cell renal cell carcinoma[J]. Nat Med, 2020, 26 (6): 909- 918.
doi: 10.1038/s41591-020-0839-y |
| 13 |
McDermott DF , Huseni MA , Atkins MB , et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma[J]. Nat Med, 2018, 24 (6): 749- 757.
doi: 10.1038/s41591-018-0053-3 |
| 14 |
Braun DA , Ishii Y , Walsh AM , et al. Clinical validation of PBRM1 alterations as a marker of immune checkpoint inhibitor response in renal cell carcinoma[J]. JAMA Oncol, 2019, 5 (11): 1631- 1633.
doi: 10.1001/jamaoncol.2019.3158 |
| 15 |
Motzer RJ , Banchereau R , Hamidi H , et al. Molecular subsets in renal cancer determine outcome to checkpoint and angiogenesis blockade[J]. Cancer cell, 2020, 38 (6): 803- 817.
doi: 10.1016/j.ccell.2020.10.011 |
| 16 |
Hwang J , Kim H , Han J , et al. Identification of survival-specific genes in clear cell renal cell carcinoma using a customized next-generation sequencing gene panel[J]. J Pers Med, 2022, 12 (1): 113.
doi: 10.3390/jpm12010113 |
| 17 |
Bui TO , Feugeas JP , Pamoukdjian F , et al. Genomics of clear-cell renal cell carcinoma: A systematic review and meta-analysis[J]. Eur Urol, 2022, 81 (4): 349- 361.
doi: 10.1016/j.eururo.2021.12.010 |
| 18 | Zhou C , Niu Y , Ma T , et al. The predictive values of ARID1A mutations for response to immune checkpoint inhibitors are varied in different types of solid tumors[J]. Cancer Res, 2021, 81 (Suppl 13): 1641. |
| 19 |
De Velasco G , Miao D , Voss MH , et al. Tumor mutational load and immune parameters across metastatic renal cell carcinoma risk groups[J]. Cancer Immunol Res, 2016, 4 (10): 820- 822.
doi: 10.1158/2326-6066.CIR-16-0110 |
| 20 |
Chen DS , Mellman I . Elements of cancer immunity and the cancer-immune set point[J]. Nature, 2017, 541 (7637): 321- 330.
doi: 10.1038/nature21349 |
| 21 |
Binnewies M , Roberts EW , Kersten K , et al. Understanding the tumor immune microenvironment (TIME) for effective therapy[J]. Nat Med, 2018, 24 (5): 541- 550.
doi: 10.1038/s41591-018-0014-x |
| [1] | 刘家骏, 刘国康, 朱玉虎. 免疫相关性重症肺炎1例[J]. 北京大学学报(医学版), 2024, 56(5): 932-937. |
| [2] | 张树栋,谢睿扬. 机器人手术时代的肾癌合并腔静脉瘤栓治疗策略[J]. 北京大学学报(医学版), 2024, 56(4): 562-564. |
| [3] | 舒帆,郝一昌,张展奕,邓绍晖,张洪宪,刘磊,王国良,田晓军,赵磊,马潞林,张树栋. 肾部分切除术治疗囊性肾癌的功能学和肿瘤学结果:单中心回顾性研究[J]. 北京大学学报(医学版), 2024, 56(4): 667-672. |
| [4] | 兰东,刘茁,李宇轩,王国良,田晓军,马潞林,张树栋,张洪宪. 根治性肾切除和静脉癌栓取出术大出血的危险因素[J]. 北京大学学报(医学版), 2023, 55(5): 825-832. |
| [5] | 时云飞,王豪杰,刘卫平,米岚,龙孟平,刘雁飞,赖玉梅,周立新,刁新婷,李向红. 血管免疫母细胞性T细胞淋巴瘤临床与分子病理学特征分析[J]. 北京大学学报(医学版), 2023, 55(3): 521-529. |
| [6] | 刘茁,朱国栋,唐世英,洪鹏,赵勋,张启鸣,李丽伟,彭冉,陈志刚,王滨帅,张丽,杨飞龙,葛力源,孙争辉,张树栋,王国良,田晓军,张洪宪,马潞林. 外科手术治疗年龄≥75岁的高龄肾细胞癌合并静脉癌栓患者的临床经验[J]. 北京大学学报(医学版), 2022, 54(4): 774-778. |
| [7] | 刘圣杰,侯惠民,吕政通,丁鑫,王璐,张磊,刘明. 双极雄激素序贯免疫检查点抑制剂治疗转移性去势抵抗性前列腺癌4例[J]. 北京大学学报(医学版), 2022, 54(4): 766-769. |
| [8] | 应沂岑,唐琦,杨恺惟,米悦,范宇,虞巍,宋毅,何志嵩,周利群,李学松. 泌尿肿瘤免疫检查点抑制剂相关性肌炎的临床特征[J]. 北京大学学报(医学版), 2022, 54(4): 644-651. |
| [9] | 顾阳春,刘颖,谢超,曹宝山. 程序性死亡蛋白-1抑制剂治疗晚期肺癌出现垂体免疫不良反应3例[J]. 北京大学学报(医学版), 2022, 54(2): 369-375. |
| [10] | 洪鹏,田晓军,赵小钰,杨飞龙,刘茁,陆敏,赵磊,马潞林. 肾移植术后双侧乳头状肾癌1例[J]. 北京大学学报(医学版), 2021, 53(4): 811-813. |
| [11] | 肖若陶,刘承,徐楚潇,何为,马潞林. 术前血小板参数与局部进展期肾细胞癌预后[J]. 北京大学学报(医学版), 2021, 53(4): 647-652. |
| [12] | 吴君怡,余淼,孙仕晨,樊壮壮,郑静蕾,张刘陶,冯海兰,刘洋,韩冬. 少汗性外胚层发育不良患者EDA基因突变检测及表型分析[J]. 北京大学学报(医学版), 2021, 53(1): 24-33. |
| [13] | 徐帅,王旸烁,李纾,刘海鹰. 肾癌及脑膜瘤术后并发吉兰-巴雷综合征1例[J]. 北京大学学报(医学版), 2019, 51(4): 775-777. |
| [14] | 李丽伟,刘茁,王国良,张华,陈文,马静,张丽,何为,马潞林,王淑敏. 肾癌伴下腔静脉瘤栓合并血栓的多种影像学比较[J]. 北京大学学报(医学版), 2019, 51(4): 678-683. |
| [15] | 唐琦,林榕城,姚林,张争,郝瀚,张崔建,蔡林,李学松,何志嵩,周利群. 肾癌术后局部复发患者的临床病理特征及预后分析[J]. 北京大学学报(医学版), 2019, 51(4): 628-631. |
|
||