北京大学学报(医学版) ›› 2021, Vol. 53 ›› Issue (4): 647-652. doi: 10.19723/j.issn.1671-167X.2021.04.004
术前血小板参数与局部进展期肾细胞癌预后
- 北京大学第三医院泌尿外科,北京 100191
Prognostic value of preoperative platelet parameters in locally advanced renal cell carcinoma
XIAO Ruo-tao,LIU Cheng,XU Chu-xiao,HE Wei,MA Lu-lin( )
)
- Department of Urology, Peking University Third Hospital, Beijing 100191, China
摘要:
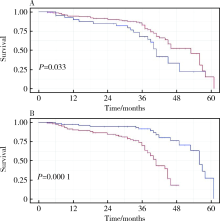
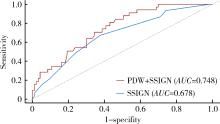
目的: 探讨术前血小板参数对局部进展期肾癌预后的预测价值,为此类患者的危险分层提供参考。方法: 选择北京大学第三医院2015年1月—2017年12月局部进展期肾癌患者进行回顾性分析,依据随访过程中肿瘤是否复发或转移分成进展组和无进展组,比较两组术前血小板参数和临床资料的差异。通过受试者特征工作曲线(receiver operating characteristic curve, ROC)确定血小板参数的最佳临界值,Kaplan-Meier生存曲线分析不同血小板参数与疾病无进展生存时间(progression-free survival, PFS)的关系。通过Cox比例风险模型进行多因素分析确定PFS的独立危险因素。采用时间依赖ROC、净重新分类指数(net reclassification index, NRI)和综合判别改善指数(integrated discrimination improvement, IDI)评估纳入血小板参数后对SSIGN模型改良情况。结果: 共有215例患者入选本研究,其中192例(89.3%)患者获得随访,中位随访时间为36个月。64例(29.8%)患者随访过程中出现疾病进展,中位PFS为46个月。进展组患者的血小板数量(platelet count, PLT)相较无进展组高[(250.72±88.59)×109/L vs. (227.27±66.94)×109/L, P=0.042],血小板分布宽度(platelet distribution width, PDW)相较无进展组低[(12.01±2.27)% vs. (13.31±2.74)%, P=0.001]。将285×109/L及12.65%作为PLT及PDW的最佳临界值,PLT≤285×109/L组患者中位PFS显著长于PLT>285×109/L组(53个月vs. 41个月,P=0.033);PDW>12.65%组患者中位PFS也显著长于PDW≤12.65%组(56个月vs. 41个月,P<0.001)。多因素分析显示术前PDW(HR=0.735, P<0.001)、细胞核分级Ⅲ~Ⅳ级(HR=2.425, P=0.001)、合并肉瘤样分化(HR=3.101, P=0.008)为PFS的独立危险因素。术前PDW联合SSIGN预后评分模型曲线下面积大于原有SSIGN模型[0.748 (95%CI:0.662~0.833) vs. 0.678 (95%CI: 0.583~0.773), P=0.193],NRI为0.262(P=0.04),IDI为 0.085(P=0.01),表明PDW纳入SSIGN模型后其预测能力提高。结论: 术前高PLT和低PDW与局部进展期肾癌不良预后相关,其中PDW是患者预后的独立危险因素,因此,术前PDW有助于对局部进展期肾癌进行危险分层。
中图分类号:
- R737.11
| [1] |
Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis [J]. Nat Rev Cancer, 2011, 11(2):123-134.
doi: 10.1038/nrc3004 |
| [2] |
Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis [J]. Cancer Cell, 2011, 20(5):576-590.
doi: 10.1016/j.ccr.2011.09.009 pmid: 22094253 |
| [3] |
Nieswandt B, Hafner M, Echtenacher B, et al. Lysis of tumor cells by natural killer cells in mice is impeded by platelets [J]. Cancer Res, 1999, 59(6):1295-1300.
pmid: 10096562 |
| [4] |
Zhu X, Cao Y, Lu P, et al. Evaluation of platelet indices as diagnostic biomarkers for colorectal cancer [J]. Sci Rep, 2018, 8(1):11814.
doi: 10.1038/s41598-018-29293-x |
| [5] |
Liu C, Zhang H, Qi Q, et al. The preoperative platelet distribution width: A predictive factor of the prognosis in patients with non-small cell lung cancer [J]. Thorac Cancer, 2020, 11(4):918-927.
doi: 10.1111/tca.v11.4 |
| [6] |
Liu S, Fang J, Jiao D, et al. Elevated platelet count predicts poor prognosis in breast cancer patients with supraclavicular lymph node metastasis [J]. Cancer Manag Res, 2020, 12(6):6069-6075.
doi: 10.2147/CMAR.S257727 |
| [7] |
Heng DY, Xie W, Regan MM, et al. External validation and comparison with other models of the international metastatic renal-cell carcinoma database consortium prognostic model: a population-based study [J]. Lancet Oncol, 2013, 14(2):141-148.
doi: 10.1016/S1470-2045(12)70559-4 |
| [8] |
Choi JY, Ko YH, Song PH. Clinical significance of preoperative thrombocytosis in patients who underwent radical nephrectomy for nonmetastatic renal cell carcinoma [J]. Investig Clin Urol, 2016, 57(5):324-329.
doi: 10.4111/icu.2016.57.5.324 |
| [9] |
Seles M, Posch F, Pichler GP, et al. Blood platelet volume represents a novel prognostic factor in patients with nonmetastatic renal cell carcinoma and improves the predictive ability of established prognostic scores [J]. J Urol, 2017, 198(6):1247-1252.
doi: 10.1016/j.juro.2017.07.036 |
| [10] |
Karakiewicz PI, Trinh QD, Lam JS, et al. Platelet count and preoperative haemoglobin do not significantly increase the performance of established predictors of renal cell carcinoma-specific mortality [J]. Eur Urol, 2007, 52(5):1428-1436.
pmid: 17420085 |
| [11] |
Frank I, Blute ML, Cheville JC, et al. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: the SSIGN score [J]. J Urol, 2002, 168(6):2395-2400.
doi: 10.1016/S0022-5347(05)64153-5 |
| [12] |
Zisman A, Pantuck AJ, Dorey F, et al. Improved prognostication of renal cell carcinoma using an integrated staging system [J]. J Clin Oncol, 2001, 19(6):1649-1657.
pmid: 11250993 |
| [13] | Amin MB, Edge SB, Greene FL, et al. AJCC cancer staging manual[M]. 8th ed. Chicago: Springer, 2017: 739-747. |
| [14] |
Ljungberg B, Bensalah K, Canfield S, et al. EAU guidelines on renal cell carcinoma: 2014 update [J]. Eur Urol, 2015, 67(5):913-924.
doi: 10.1016/j.eururo.2015.01.005 pmid: 25616710 |
| [15] |
Moch H, Cubilla AL, Humphrey PA, et al. The 2016 WHO classification of tumours of the urinary system and male genital organs-part A: renal, penile, and testicular tumours [J]. Eur Urol, 2016, 70(1):93-105.
doi: 10.1016/j.eururo.2016.02.029 |
| [16] |
Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021 [J]. CA Cancer J Clin, 2021, 71(1):7-33.
doi: 10.3322/caac.v71.1 |
| [17] |
Haas NB, Manola J, Dutcher JP, et al. Adjuvant treatment for high-risk clear cell renal cancer: updated results of a high-risk subset of the ASSURE randomized trial [J]. JAMA Oncol, 2017, 3(9):1249-1252.
doi: 10.1001/jamaoncol.2017.0076 |
| [18] |
Motzer RJ, Haas NB, Donskov F, et al. Randomized phase Ⅲ trial of adjuvant pazopanib versus placebo after nephrectomy in patients with localized or locally advanced renal cell carcinoma [J]. J Clin Oncol, 2017, 35(35):3916-3923.
doi: 10.1200/JCO.2017.73.5324 |
| [19] |
Motzer RJ, Ravaud A, Patard JJ, et al. Adjuvant sunitinib for high-risk renal cell carcinoma after nephrectomy: subgroup analyses and updated overall survival results [J]. Eur Urol, 2018, 73(1):62-68.
doi: 10.1016/j.eururo.2017.09.008 |
| [20] |
Leibovich BC, Blute ML, Cheville JC, et al. Prediction of progression after radical nephrectomy for patients with clear cell renal cell carcinoma: a stratification tool for prospective clinical trials [J]. Cancer, 2003, 97(7):1663-1671.
pmid: 12655523 |
| [21] |
Karakiewicz PI, Briganti A, Chun FK, et al. Multi-institutional validation of a new renal cancer-specific survival nomogram [J]. J Clin Oncol, 2007, 25(11):1316-1322.
pmid: 17416852 |
| [22] |
Correa AF, Jegede O, Haas NB, et al. Predicting renal cancer recurrence: defining limitations of existing prognostic models with prospective trial-based validation [J]. J Clin Oncol, 2019, 37(23):2062-2071.
doi: 10.1200/JCO.19.00107 |
| [23] |
Xiao R, Xu C, He W, et al. Preoperative anaemia and thrombocytosis predict adverse prognosis in non-metastatic renal cell carcinoma with tumour thrombus [J]. BMC Urol, 2021, 21(1):31.
doi: 10.1186/s12894-021-00796-6 |
| [24] |
Yue CX, Liu YX, Yun ZY, et al. Decreased platelet distribution width predicts a worse prognosis in patients undergoing surgical resection for hepatocellular carcinoma [J]. Cancer Biomark, 2019, 26(3):361-366.
doi: 10.3233/CBM-190474 |
| [25] |
Chen H, Wu Q, Zhang Y, et al. Nomograms based on the novel platelet index score predict postoperative prognosis in endometrial cancer [J]. Gynecol Oncol, 2020, 158(3):689-697.
doi: S0090-8258(20)31125-2 pmid: 32507649 |
| [26] |
Kawakita Y, Motoyama S, Sato Y, et al. Prognostic significance of combined platelet distribution width and C-reactive protein score in esophageal cancer [J]. Anticancer Res, 2020, 40(10):5715-5725.
doi: 10.21873/anticanres.14586 pmid: 32988897 |
| [27] | 蒋慧云, 李小毛, 王佳, 等. 术前血小板分布宽度在子宫内膜癌诊断预测中的价值 [J]. 实用医学杂志, 2018, 34(7):1188-1190. |
| [28] |
Vagdatli E, Gounari E, Lazaridou E, et al. Platelet distribution width: a simple, practical and specific marker of activation of coagulation [J]. Hippokratia, 2010, 14(1):28-32.
pmid: 20411056 |
| [29] | 张翔, 庄瑞. 血小板分布宽度对鼻咽癌患者预后的影响 [J]. 国际肿瘤学杂志, 2018, 45(5):257-261. |
| [30] |
Huang Y, Cui MM, Huang YX, et al. Preoperative platelet distribution width predicts breast cancer survival [J]. Cancer Biomark, 2018, 23(2):205-211.
doi: 10.3233/CBM-181267 pmid: 30198864 |
| [31] | 张林楠, 刘玉峰, 苏淑芳, 等. 血小板分布宽度对神经母细胞瘤预后的预测价值 [J]. 中华实用儿科临床杂志, 2020, 35(6):440-444. |
| [1] | 张树栋,谢睿扬. 机器人手术时代的肾癌合并腔静脉瘤栓治疗策略[J]. 北京大学学报(医学版), 2024, 56(4): 562-564. |
| [2] | 欧俊永,倪坤明,马潞林,王国良,颜野,杨斌,李庚午,宋昊东,陆敏,叶剑飞,张树栋. 肌层浸润性膀胱癌合并中高危前列腺癌患者的预后因素[J]. 北京大学学报(医学版), 2024, 56(4): 582-588. |
| [3] | 刘帅,刘磊,刘茁,张帆,马潞林,田晓军,侯小飞,王国良,赵磊,张树栋. 伴静脉癌栓的肾上腺皮质癌的临床治疗及预后[J]. 北京大学学报(医学版), 2024, 56(4): 624-630. |
| [4] | 虞乐,邓绍晖,张帆,颜野,叶剑飞,张树栋. 具有低度恶性潜能的多房囊性肾肿瘤的临床病理特征及预后[J]. 北京大学学报(医学版), 2024, 56(4): 661-666. |
| [5] | 舒帆,郝一昌,张展奕,邓绍晖,张洪宪,刘磊,王国良,田晓军,赵磊,马潞林,张树栋. 肾部分切除术治疗囊性肾癌的功能学和肿瘤学结果:单中心回顾性研究[J]. 北京大学学报(医学版), 2024, 56(4): 667-672. |
| [6] | 周泽臻,邓绍晖,颜野,张帆,郝一昌,葛力源,张洪宪,王国良,张树栋. 非转移性T3a肾细胞癌患者3年肿瘤特异性生存期预测[J]. 北京大学学报(医学版), 2024, 56(4): 673-679. |
| [7] | 方杨毅,李强,黄志高,陆敏,洪锴,张树栋. 睾丸鞘膜高分化乳头状间皮肿瘤1例[J]. 北京大学学报(医学版), 2024, 56(4): 741-744. |
| [8] | 曾媛媛,谢云,陈道南,王瑞兰. 脓毒症患者发生正常甲状腺性病态综合征的相关因素[J]. 北京大学学报(医学版), 2024, 56(3): 526-532. |
| [9] | 苏俊琪,王晓颖,孙志强. 舌鳞状细胞癌根治性切除术后患者预后预测列线图的构建与验证[J]. 北京大学学报(医学版), 2024, 56(1): 120-130. |
| [10] | 李建斌,吕梦娜,池强,彭一琳,刘鹏程,吴锐. 干燥综合征患者发生重症新型冠状病毒肺炎的早期预测[J]. 北京大学学报(医学版), 2023, 55(6): 1007-1012. |
| [11] | 刘欢锐,彭祥,李森林,苟欣. 基于HER-2相关基因构建风险模型用于膀胱癌生存预后评估[J]. 北京大学学报(医学版), 2023, 55(5): 793-801. |
| [12] | 薛子璇,唐世英,邱敏,刘承,田晓军,陆敏,董靖晗,马潞林,张树栋. 青年肾肿瘤伴瘤栓的临床病理特征及预后分析[J]. 北京大学学报(医学版), 2023, 55(5): 802-811. |
| [13] | 兰东,刘茁,李宇轩,王国良,田晓军,马潞林,张树栋,张洪宪. 根治性肾切除和静脉癌栓取出术大出血的危险因素[J]. 北京大学学报(医学版), 2023, 55(5): 825-832. |
| [14] | 卢汉,张建运,杨榕,徐乐,李庆祥,郭玉兴,郭传瑸. 下颌牙龈鳞状细胞癌患者预后的影响因素[J]. 北京大学学报(医学版), 2023, 55(4): 702-707. |
| [15] | 时云飞,王豪杰,刘卫平,米岚,龙孟平,刘雁飞,赖玉梅,周立新,刁新婷,李向红. 血管免疫母细胞性T细胞淋巴瘤临床与分子病理学特征分析[J]. 北京大学学报(医学版), 2023, 55(3): 521-529. |
|
||