北京大学学报(医学版) ›› 2021, Vol. 53 ›› Issue (4): 744-749. doi: 10.19723/j.issn.1671-167X.2021.04.021
钙结合蛋白在健康牙周组织和实验性牙周炎组织的表达分布
- 1.北京大学口腔医学院·口腔医院,牙周科 国家口腔医学中心 国家口腔疾病临床医学研究中心 口腔数字化医疗技术和材料国家工程实验室,北京 100081
2.天津医科大学口腔医院牙周科,天津 300070
Expression and distribution of calprotectin in healthy and inflamed periodontal tissues
GAO Hong-yu1,2,MENG Huan-xin1,Δ( ),HOU Jian-xia1,HUANG Bao-xin1,LI Wei1
),HOU Jian-xia1,HUANG Bao-xin1,LI Wei1
- 1. Department of Periodontology, Peking University School and Hospital of Stomatology & National Center of Stomatology & National Clinical Research Center for Oral Diseases & National Engineering Laboratory for Digital and Material Technology of Stomatology, Beijing 100081, China
2. Department of Periodontology, Stomatological Hospital of Tianjin Medical University, Tianjin 300070, China
摘要:
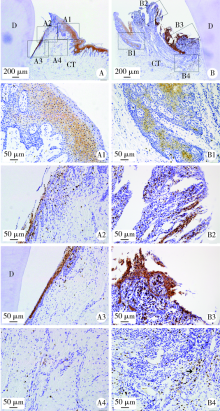
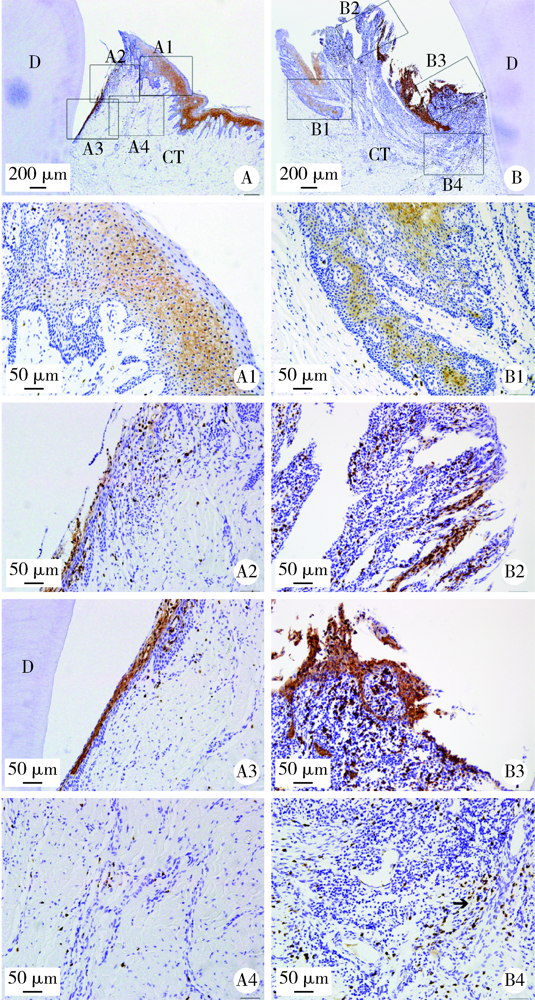
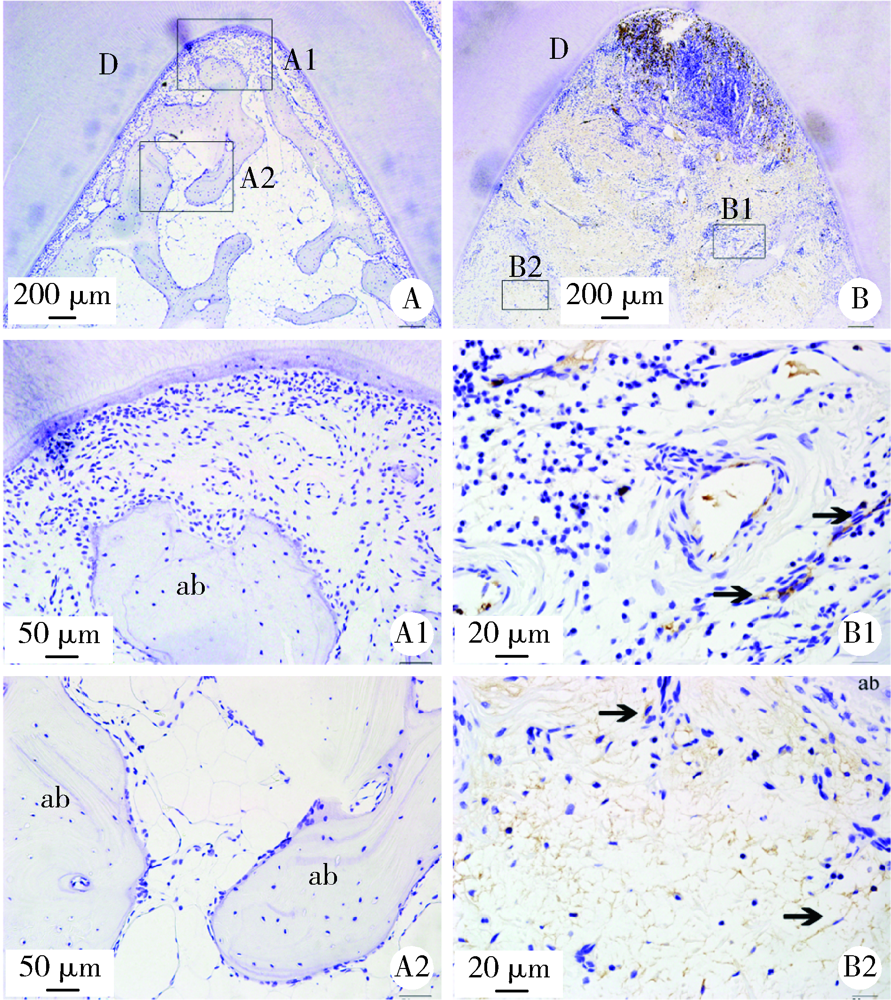
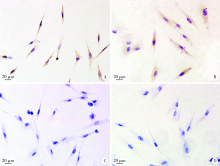
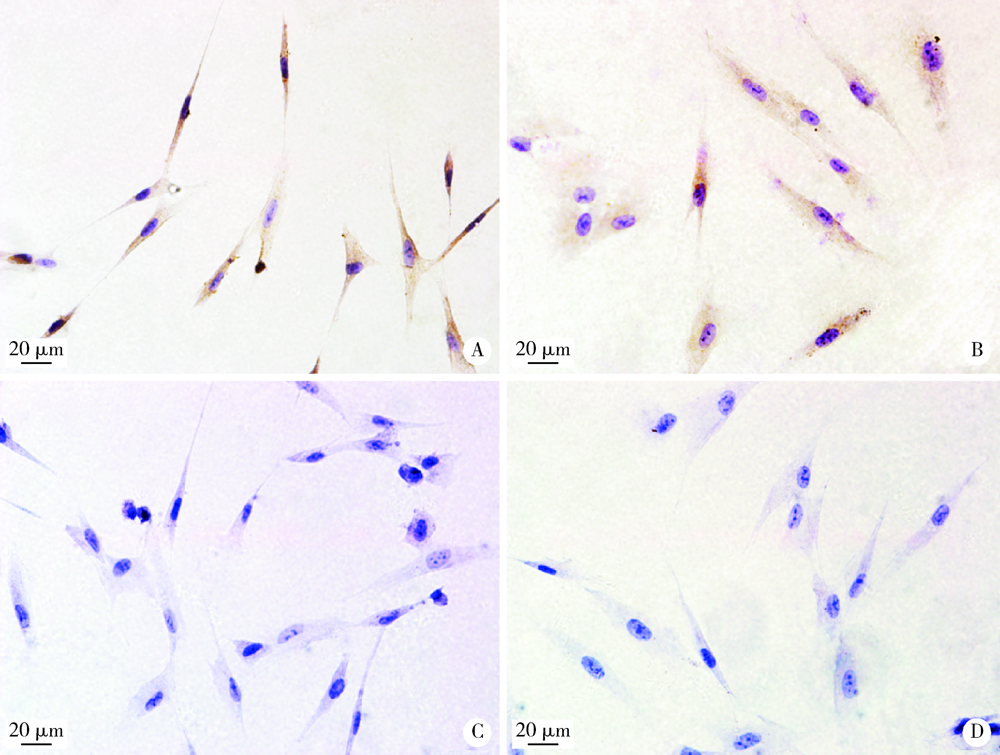
目的: 观察比格犬相对健康的牙周组织和实验性牙周炎的牙周组织标本中钙结合蛋白的表达分布和细胞定位,探讨其与牙周炎症的关系和可能发挥的作用。方法: 结扎法诱导建立比格犬下颌第二磨牙实验性牙周炎模型,对侧同名牙作为健康对照,诱导12周后处死,组织脱矿后常规制作连续切片,免疫组织化学法检测钙结合蛋白在比格犬健康和实验性牙周炎的组织标本中的表达和分布,明确细胞定位;免疫细胞化学法检测体外培养的原代人牙周组织细胞中钙结合蛋白的表达。结果: 在健康牙龈组织中,钙结合蛋白表达于牙龈上皮、中性粒细胞,且在结合上皮处呈强阳性表达;牙周炎症病损中,牙龈上皮细胞钙结合蛋白表达水平上调,存在强表达钙结合蛋白的中性粒细胞大量浸润,成纤维样细胞(牙龈结缔组织、牙周膜组织)、骨髓成纤维细胞、微血管内皮细胞存在钙结合蛋白的诱导表达;在体外培养的人牙周膜细胞、人牙龈成纤维细胞中均检测到钙结合蛋白的表达。结论: 钙结合蛋白组成性表达于牙龈上皮细胞、中性粒细胞,可能对维持牙周组织内环境稳定和完整性发挥重要作用。牙周炎症诱导牙龈成纤维细胞、牙周膜细胞、骨髓成纤维细胞和血管内皮细胞表达的钙结合蛋白可能在抗微生物感染、促炎症细胞迁移等方面发挥作用。
中图分类号:
- R781.4
| [1] |
Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation [J]. Nat Rev Immunol, 2015, 15(1):30-44.
doi: 10.1038/nri3785 pmid: 25534621 |
| [2] |
Page RC, Kornman KS. The pathogenesis of human periodontitis: an introduction [J]. Periodontol 2000, 1997, 14(1):9-11.
doi: 10.1111/prd.1997.14.issue-1 |
| [3] | Di Benedetto A, Gigante I, Colucci S, et al. Periodontal disease: linking the primary inflammation to bone loss [J/OL]. Clin Dev Immunol, 2013, 2013: 503754(2013-05-23)[2019-12-01]. https//www.hindawi.com/journals/ . |
| [4] |
Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation [J]. Nat Rev Immunol, 2013, 13(3):159-175.
doi: 10.1038/nri3399 pmid: 23435331 |
| [5] |
Jin L. An update on innate defense molecules of human gingiva [J]. Periodontol 2000, 2011, 56(1):125-142.
doi: 10.1111/prd.2011.56.issue-1 |
| [6] |
Goyette J, Geczy CL. Inflammation-associated S100 proteins: new mechanisms that regulate function [J]. Amino Acids, 2011, 41(4):821-842.
doi: 10.1007/s00726-010-0528-0 |
| [7] |
Lundy FT, Chalk R, Lamey PJ, et al. Identification of MRP-8 (calgranulin A) as a major responsive protein in chronic periodontitis [J]. J Pathol, 2000, 192(4):540-544.
pmid: 11113873 |
| [8] |
Lundy FT, Chalk R, Lamey PJ, et al. Quantitative analysis of MRP-8 in gingival crevicular fluid in periodontal health and disease using microbore HPLC [J]. J Clin Periodontol, 2001, 28(12):1172-1177.
pmid: 11737516 |
| [9] |
Kido J, Nakamura T, Kido R, et al. Calprotectin in gingival crevicular fluid correlates with clinical and biochemical markers of periodontal disease [J]. J Clin Periodontol, 1999, 26(10):653-657.
pmid: 10522776 |
| [10] |
Andersen E, Dessaix IM, Perneger T, et al. Myeloid-related protein (MRP8/14) expression in gingival crevice fluid in periodontal health and disease and after treatment [J]. J Periodontal Res, 2010, 45(4):458-463.
doi: 10.1111/j.1600-0765.2009.01257.x pmid: 20337885 |
| [11] |
Ren XY, Xu L, Meng HX, et al. Family-based association analysis of S100A8 genetic polymorphisms with aggressive periodontitis [J]. J Periodontal Res, 2009, 44(2):184-192.
doi: 10.1111/j.1600-0765.2008.01103.x pmid: 19210342 |
| [12] |
Li QY, Meng HX, Zhang L, et al. Correlation between single nucleotide polymorphisms in a calprotectin subunit gene and risk of periodontitis in a Chinese population [J]. Ann Hum Genet, 2007, 71(Pt 3):312-324.
doi: 10.1111/ahg.2007.71.issue-3 |
| [13] |
Sun XJ, Meng HX, Shi D, et al. Analysis of plasma calprotectin and polymorphisms of S100A8 in patients with aggressive periodontitis [J]. J Periodontal Res, 2011, 46(3):354-360.
doi: 10.1111/j.1600-0765.2011.01350.x pmid: 21463326 |
| [14] | 孙晓军, 孟焕新, 释栋, 等. 侵袭性牙周炎患者血浆钙结合蛋白与血液中性粒细胞数相关性的研究 [J]. 中华口腔医学杂志, 2014, 49(11):649-651. |
| [15] |
Suryono, Kido J, Hayashi N, et al. Effect of porphyromonas gingivalis lipopolysaccharide, tumor necrosis factor-alpha, and interleukin-1beta on calprotectin release in human monocytes [J]. J Periodontol, 2003, 74(12):1719-1724.
doi: 10.1902/jop.2003.74.12.1719 pmid: 29539076 |
| [16] |
Schlegel Gomez R, Langer P, Pelka M, et al. Variational expression of functionally different macrophage markers (27E10, 25F9, RM3/1) in normal gingiva and inflammatory periodontal disease [J]. J Clin Periodontol, 1995, 22(5):341-346.
pmid: 7541406 |
| [17] |
Li W, Huang B, Liu K, et al. Up-regulated leptin in periodontitis promotes inflammatory cytokine expression in periodontal ligament cells [J]. J Periodontol, 2015, 86(7):917-926.
doi: 10.1902/jop.2015.150030 |
| [18] |
Gao H, Hou J, Meng H, et al. Proinflammatory effects and mechanisms of calprotectin on human gingival fibroblasts [J]. J Periodontal Res, 2017, 52(6):975-983.
doi: 10.1111/jre.12465 pmid: 28643937 |
| [19] |
Gao H, Zhang X, Zheng Y, et al. S100A9-induced release of interleukin (IL)-6 and IL-8 through toll-like receptor 4 (TLR4) in human periodontal ligament cells [J]. Mol Immunol, 2015, 67(2 Pt B):223-232.
doi: 10.1016/j.molimm.2015.05.014 |
| [20] |
Hiroshima Y, Bando M, Kataoka M, et al. Regulation of antimicrobial peptide expression in human gingival keratinocytes by interleukin-1alpha [J]. Arch Oral Biol, 2011, 56(8):761-767.
doi: 10.1016/j.archoralbio.2011.01.004 pmid: 21316034 |
| [21] |
Hsu K, Champaiboon C, Guenther BD, et al. Anti-infective protective properties of S100 calgranulins [J]. Antiinflamm Antiallergy Agents Med Chem, 2009, 8(4):290-305.
doi: 10.2174/187152309789838975 |
| [22] |
Jonsson D, Nebel D, Bratthall G, et al. The human periodontal ligament cell: a fibroblast-like cell acting as an immune cell [J]. J Periodontal Res, 2011, 46(2):153-157.
doi: 10.1111/jre.2011.46.issue-2 |
| [23] |
Ara T, Kurata K, Hirai K, et al. Human gingival fibroblasts are critical in sustaining inflammation in periodontal disease [J]. J Periodontal Res, 2009, 44(1):21-27.
doi: 10.1111/jre.2009.44.issue-1 |
| [24] |
Nisapakultorn K, Ross KF, Herzberg MC. Calprotectin expression in vitro by oral epithelial cells confers resistance to infection by porphyromonas gingivalis [J]. Infect Immun, 2001, 69(7):4242-4247.
pmid: 11401960 |
| [25] |
Yen T, Harrison CA, Devery JM, et al. Induction of the S100 chemotactic protein, CP-10, in murine microvascular endothelial cells by proinflammatory stimuli [J]. Blood, 1997, 90(12):4812-4821.
pmid: 9389698 |
| [1] | 胡玉如,刘娟,李文静,赵亦兵,李启强,路瑞芳,孟焕新. Ⅲ期或Ⅳ期牙周炎患者龈沟液中有机酸浓度与牙周炎的关系[J]. 北京大学学报(医学版), 2024, 56(2): 332-337. |
| [2] | 张晗,秦亦瑄,韦帝远,韩劼. 牙周炎患者种植修复维护治疗依从性的影响因素[J]. 北京大学学报(医学版), 2024, 56(1): 39-44. |
| [3] | 殳畅,韩烨,孙雨哲,杨再目,侯建霞. Ⅲ期牙周炎患者牙周基础治疗前后炎症性贫血相关指标的变化[J]. 北京大学学报(医学版), 2024, 56(1): 45-50. |
| [4] | 裴喜燕,阳雯,欧阳翔英,孙凤. 牙周内窥镜下根面清创与牙周翻瓣术疗效比较[J]. 北京大学学报(医学版), 2023, 55(4): 716-720. |
| [5] | 温静,欧阳翔英,裴喜燕,邱善湧,刘健如,刘文逸,曹采方. 重度牙周炎患者4年自然进展失牙的多因素分析[J]. 北京大学学报(医学版), 2023, 55(1): 70-77. |
| [6] | 朱小玲,李文静,王宪娥,宋文莉,徐莉,张立,冯向辉,路瑞芳,释栋,孟焕新. 细胞色素B-245α链及胆固醇酯转运蛋白基因多态性与广泛型侵袭性牙周炎易感性的关系[J]. 北京大学学报(医学版), 2022, 54(1): 18-22. |
| [7] | 徐欣然,霍芃呈,和璐,孟焕新,朱筠轩,靳东思奇. 伴与不伴糖尿病的牙周炎患者牙周基础治疗的疗效比较及其与白细胞水平的相关分析[J]. 北京大学学报(医学版), 2022, 54(1): 48-53. |
| [8] | 刘建,王宪娥,吕达,乔敏,张立,孟焕新,徐莉,毛铭馨. 广泛型侵袭性牙周炎患者牙根形态异常与相关致病基因的关联[J]. 北京大学学报(医学版), 2021, 53(1): 16-23. |
| [9] | 郜洪宇,徐菁玲,孟焕新,和璐,侯建霞. 牙周基础治疗对2型糖尿病伴慢性牙周炎患者红细胞、血小板相关指标的影响[J]. 北京大学学报(医学版), 2020, 52(4): 750-754. |
| [10] | 闫乐,王宪娥,詹雅琳,苗莉莉,韩烨,张楚人,岳兆国,胡文杰,侯建霞. 超声龈下清创联合手工根面平整术治疗重度牙周炎的临床效果[J]. 北京大学学报(医学版), 2020, 52(1): 64-70. |
| [11] | 张勇,刘畅,陈彬,陈帆,段晋瑜,张孟钧,焦剑. 糖尿病前期患者糖代谢异常与慢性牙周炎的相关性[J]. 北京大学学报(医学版), 2020, 52(1): 71-76. |
| [12] | 朱洁,李建红,袁婷婷,和璐,梁宇红. 绝经期妇女牙周状况与骨质密度的相关性分析[J]. 北京大学学报(医学版), 2019, 51(6): 1115-1118. |
| [13] | 石姝雯,孟洋,焦剑,李文静,孟焕新,栾庆先,王万春. 根分叉病变患牙经牙周非手术治疗后5年失牙状况及多因素分析[J]. 北京大学学报(医学版), 2019, 51(5): 913-918. |
| [14] | 杜仁杰,焦剑,周彦恒,施捷. 侵袭性牙周炎患者正畸前后的咬合变化[J]. 北京大学学报(医学版), 2019, 51(5): 919-924. |
| [15] | 刘园,栾庆先. 北京石景山社区中老年人群慢性牙周炎和颈动脉内膜中层厚度的相关性[J]. 北京大学学报(医学版), 2018, 50(2): 264-270. |
|
||