北京大学学报(医学版) ›› 2022, Vol. 54 ›› Issue (6): 1074-1078. doi: 10.19723/j.issn.1671-167X.2022.06.003
CCL28-CCR10通路在类风湿关节炎单核细胞迁移中的作用
程昉1,*( ),杨邵英2,房星星3,王璇3,赵福涛1,*(
),杨邵英2,房星星3,王璇3,赵福涛1,*( )
)
- 1. 上海交通大学医学院附属第九人民医院风湿免疫科,上海 201999
2. 上海交通大学医学院附属仁济医院风湿科,上海 200001
3. 同济大学附属同济医院风湿免疫科,上海 200065
Role of the CCL28-CCR10 pathway in monocyte migration in rheumatoid arthritis
Fang CHENG1,*( ),Shao-ying YANG2,Xing-xing FANG3,Xuan WANG3,Fu-tao ZHAO1,*(
),Shao-ying YANG2,Xing-xing FANG3,Xuan WANG3,Fu-tao ZHAO1,*( )
)
- 1. Department of Rheumatology and Immunology, Ninth People's Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 201999, China
2. Department of Rheumatology, Renji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200001, China
3. Department of Rheumatology and Immunology, Tongji Hospital, Tongji University, Shanghai 200065, China
摘要:
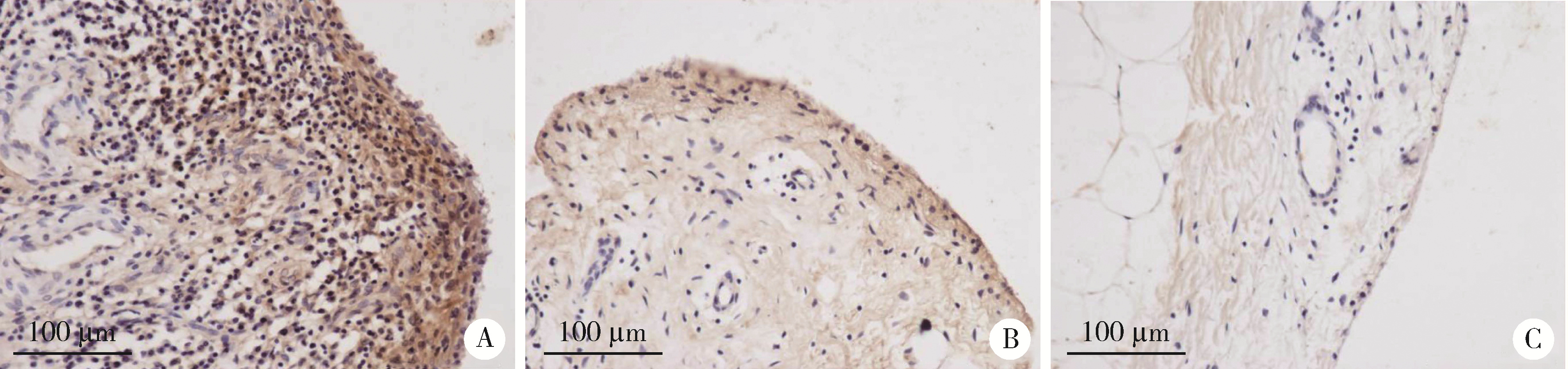
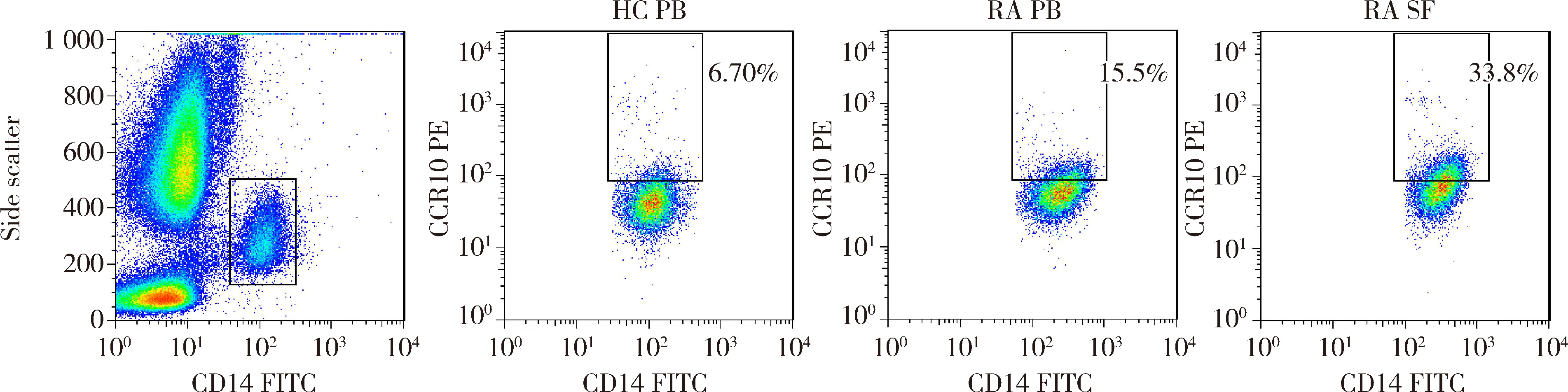
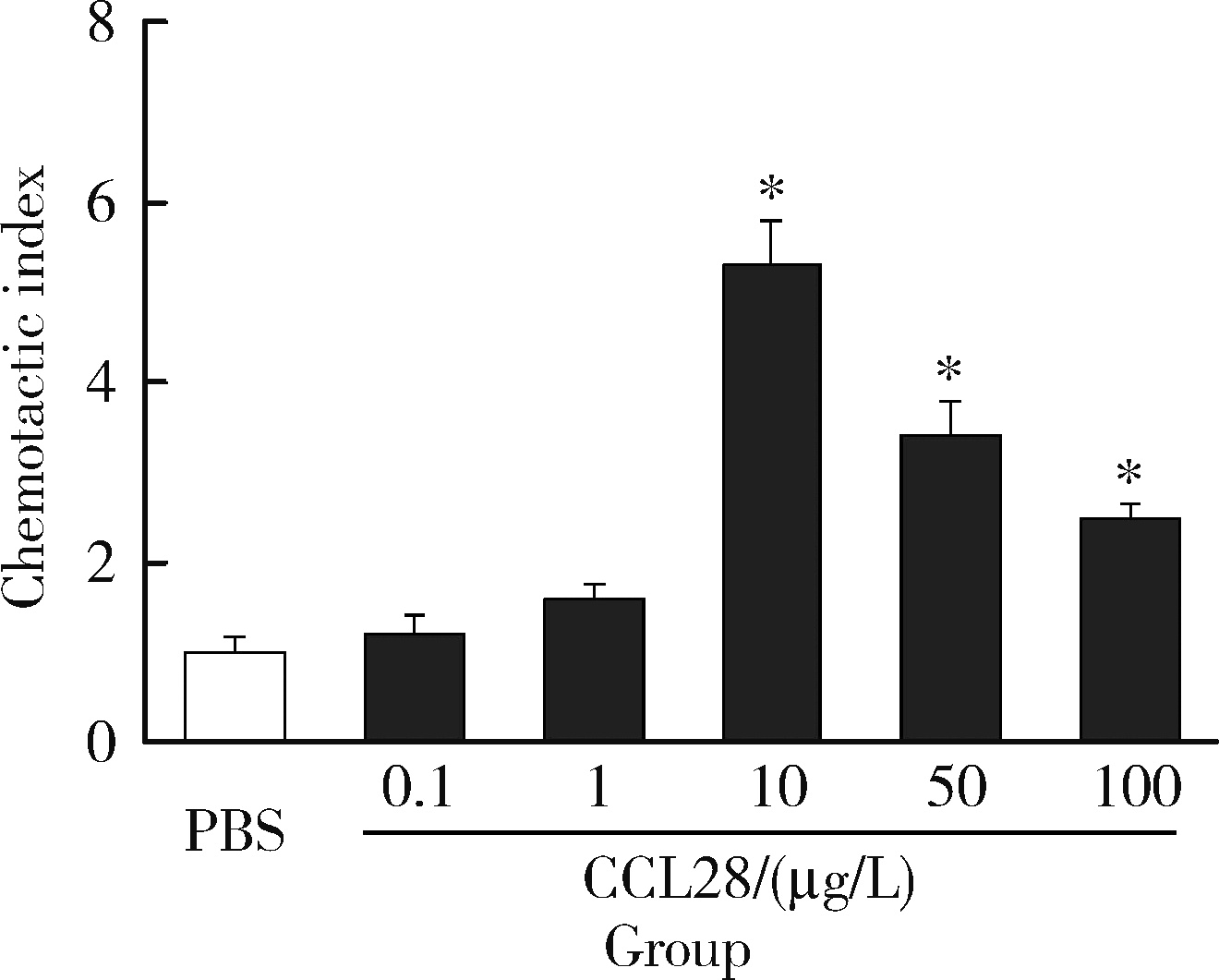
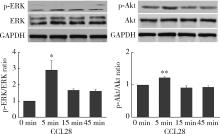
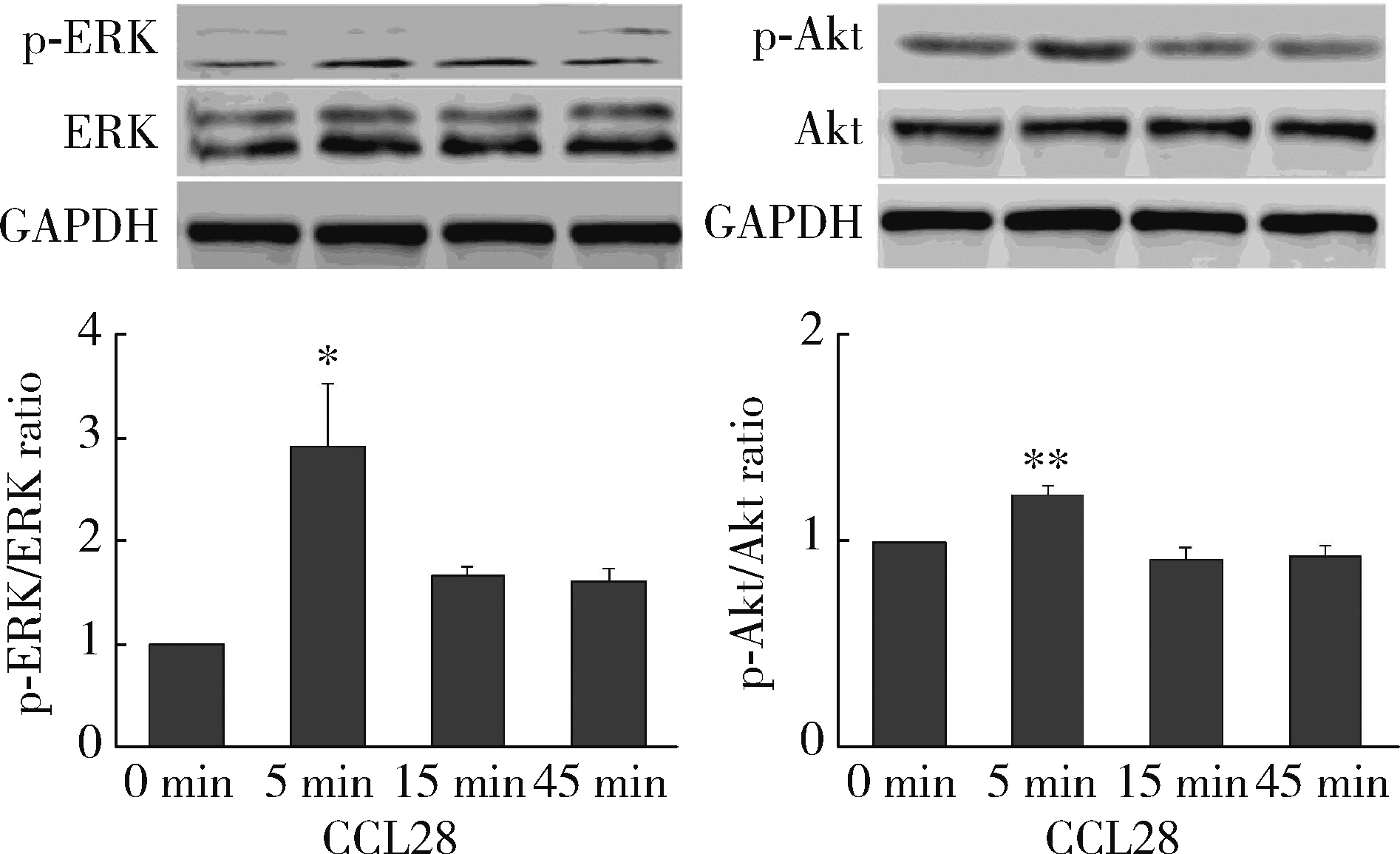
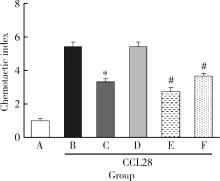
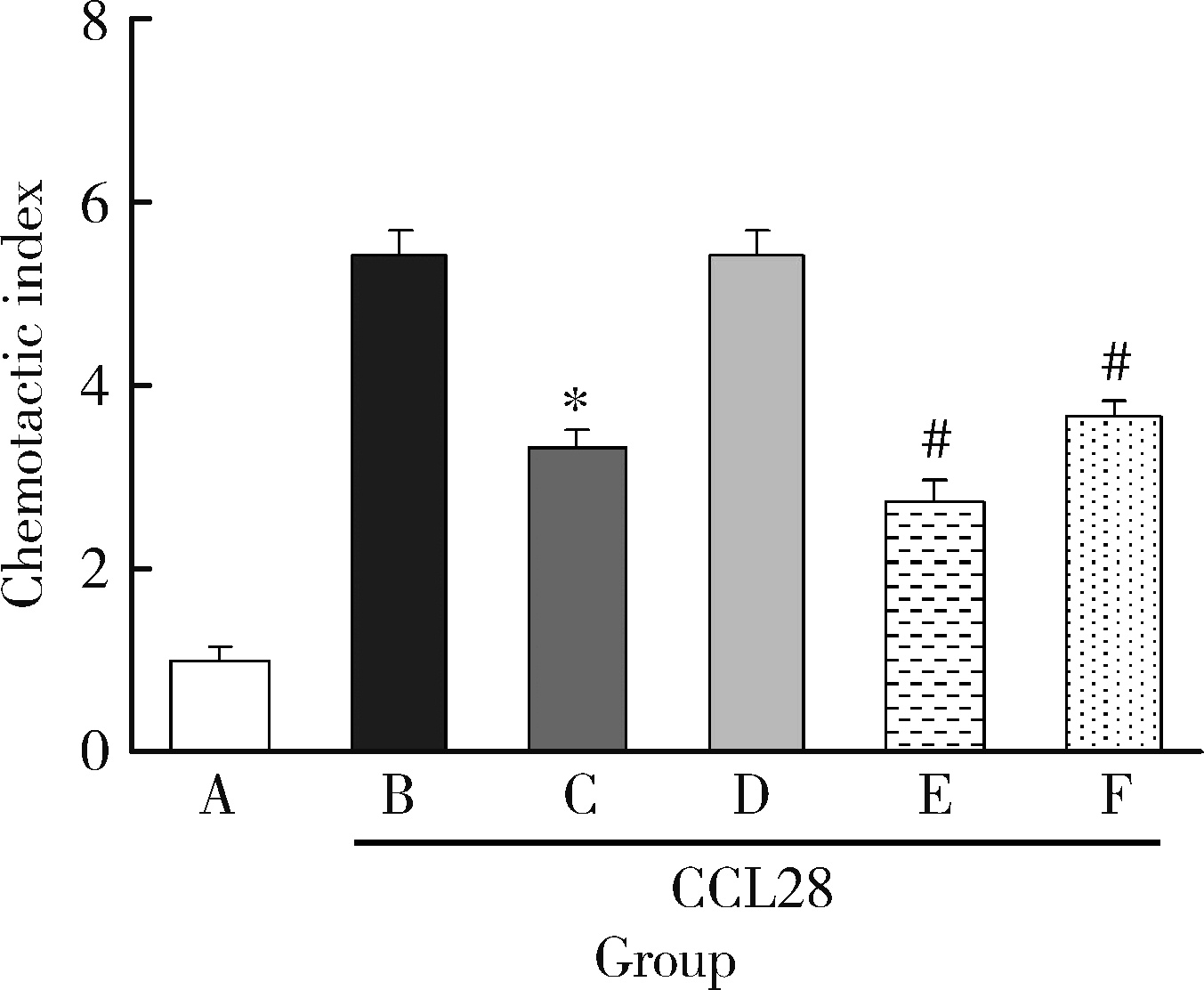
目的: 观察类风湿关节炎(rheumatoid arthritis,RA)患者关节中单核/巨噬细胞趋化因子受体CCR10的表达,探讨趋化因子CCL28与其受体CCR10在RA单核细胞迁移中的作用及机制。方法: 采用免疫组织化学法分析8例RA患者、4例骨关节炎(osteoarthritis,OA)患者和4例正常对照者滑膜组织中CCR10的表达并进行细胞染色评分(0~5分),流式细胞术检测26例RA患者和20例健康对照者外周血、15例RA患者滑液CD14+单核细胞中CCR10阳性细胞比例,Transwell迁移实验检测CCL28对RA和健康对照单核细胞的趋化性,Western blotting检测CCL28干预RA单核细胞的细胞外信号调节激酶(extracellular signal-regulated kinase,ERK)、蛋白激酶B(protein kinase B,Akt)通路磷酸化。结果: CCR10表达在RA滑膜衬里层细胞及衬里下层的巨噬细胞、血管内皮细胞、淋巴细胞;RA滑膜衬里层细胞和衬里下层巨噬细胞的CCR10表达明显高于OA和正常对照的滑膜(P均 < 0.01)。RA患者外周血CD14+单核细胞表达CCR10明显高于健康对照者[(15.6±3.0)% vs. (7.7±3.8)%, P < 0.01];RA患者滑液单核细胞CCR10的表达为(32.0±15.0)%,明显高于RA外周血(P < 0.01)。体外实验中,10~100 μg/L的CCL28能有效诱导RA和健康对照外周血CD14+单核细胞迁移(P均 < 0.01);抗CCR10单抗能明显抑制CCL28对RA单核细胞的趋化(P < 0.01)。CCL28干预RA单核细胞明显增加ERK和Akt的磷酸化(P均 < 0.05);ERK抑制剂(U0126)、磷脂酰肌醇3-激酶(phosphatidylinositol 3-kinase,PI3K)抑制剂(LY294002)可明显降低CCL28诱导的RA单核细胞迁移(P均 < 0.01)。结论: RA患者外周血、滑液及滑膜单核/巨噬细胞CCR10表达增高,CCL28与CCR10结合并通过激活ERK、PI3K/Akt信号通路促使RA单核细胞迁移;CCL28-CCR10通路可能参与招募单核细胞进入RA关节,从而促进滑膜炎症和骨破坏。
中图分类号:
- R593.22
| 1 |
Sparks JA . Rheumatoid arthritis[J]. Ann Intern Med, 2019, 170 (1): ITC1- ITC16.
doi: 10.7326/AITC201901010 |
| 2 |
Jang S , Kwon EJ , Lee JJ . Rheumatoid arthritis: Pathogenic roles of diverse immune cells[J]. Int J Mol Sci, 2022, 23 (2): 905.
doi: 10.3390/ijms23020905 |
| 3 |
Szekanecz Z , Koch AE . Successes and failures of chemokine-pathway targeting in rheumatoid arthritis[J]. Nat Rev Rheumatol, 2016, 12 (1): 5- 13.
doi: 10.1038/nrrheum.2015.157 |
| 4 |
Pan J , Kunkel EJ , Gosslar U , et al. A novel chemokine ligand for CCR10 and CCR3 expressed by epithelial cells in mucosal tissues[J]. J Immunol, 2000, 165 (6): 2943- 2949.
doi: 10.4049/jimmunol.165.6.2943 |
| 5 |
Wang W , Soto H , Oldham ER , et al. Identification of a novel chemokine (CCL28), which binds CCR10 (GPR2)[J]. J Biol Chem, 2000, 275 (29): 22313- 22323.
doi: 10.1074/jbc.M001461200 |
| 6 |
Mohan T , Deng L , Wang BZ . CCL28 chemokine: An anchoring point bridging innate and adaptive immunity[J]. Int Immuno-pharmacol, 2017, 51, 165- 170.
doi: 10.1016/j.intimp.2017.08.012 |
| 7 |
Chen Z , Kim SJ , Essani AB , et al. Characterising the expression and function of CCL28 and its corresponding receptor, CCR10, in RA pathogenesis[J]. Ann Rheum Dis, 2015, 74 (10): 1898- 1906.
doi: 10.1136/annrheumdis-2013-204530 |
| 8 |
Miyazaki Y , Nakayamada S , Kubo S , et al. Th22 cells promote osteoclast differentiation via production of IL-22 in rheumatoid arthritis[J]. Front Immunol, 2018, 9, 2901.
doi: 10.3389/fimmu.2018.02901 |
| 9 |
Aletaha D , Neogi T , Silman AJ , et al. 2010 rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative[J]. Arthritis Rheum, 2010, 62 (9): 2569- 2581.
doi: 10.1002/art.27584 |
| 10 |
Rana AK , Li Y , Dang Q , et al. Monocytes in rheumatoid arthritis: Circulating precursors of macrophages and osteoclasts and, their heterogeneity and plasticity role in RA pathogenesis[J]. Int Immunopharmacol, 2018, 65, 348- 359.
doi: 10.1016/j.intimp.2018.10.016 |
| 11 |
Park J , Zhang X , Lee SK , et al. CCL28-induced RARβ expression inhibits oral squamous cell carcinoma bone invasion[J]. J Clin Invest, 2019, 129 (12): 5381- 5399.
doi: 10.1172/JCI125336 |
| 12 |
Facciabene A , Peng X , Hagemann IS , et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells[J]. Nature, 2011, 475 (7355): 226- 230.
doi: 10.1038/nature10169 |
| 13 |
Wu Q , Chen JX , Chen Y , et al. The chemokine receptor CCR10 promotes inflammation-driven hepatocarcinogenesis via PI3K/Akt pathway activation[J]. Cell Death Dis, 2018, 9 (2): 232.
doi: 10.1038/s41419-018-0267-9 |
| [1] | 刘东武, 陈杰, 高明利, 于静. 类风湿关节炎伴发淋巴结Castleman样病理改变1例[J]. 北京大学学报(医学版), 2024, 56(5): 928-931. |
| [2] | 黄会娜,赵静,赵祥格,白自然,李霞,王冠. 乳酸对类风湿关节炎患者外周血CD4+T细胞亚群的调控作用[J]. 北京大学学报(医学版), 2024, 56(3): 519-525. |
| [3] | 汤晓菲,李永红,丁秋玲,孙卓,张阳,王育梅,田美伊,刘坚. 类风湿关节炎患者下肢深静脉血栓发病率及危险因素[J]. 北京大学学报(医学版), 2024, 56(2): 279-283. |
| [4] | 邹雪,白小娟,张丽卿. 艾拉莫德联合托法替布治疗难治性中重度类风湿关节炎的疗效[J]. 北京大学学报(医学版), 2023, 55(6): 1013-1021. |
| [5] | 吴琦,蔡月明,何娟,黄文蒂,王庆文. 血脂异常与类风湿关节炎肺间质病变的相关性分析[J]. 北京大学学报(医学版), 2023, 55(6): 982-992. |
| [6] | 张警丰,金银姬,魏慧,姚中强,赵金霞. 体重指数与类风湿关节炎临床特征的相关性分析[J]. 北京大学学报(医学版), 2023, 55(6): 993-999. |
| [7] | 金银姬,孙琳,赵金霞,刘湘源. 血清IgA型抗鼠科肉瘤病毒癌基因同源物B1抗体在类风湿关节炎中的意义[J]. 北京大学学报(医学版), 2023, 55(4): 631-635. |
| [8] | 蔡文心,李仕成,刘一鸣,梁如玉,李静,郭建萍,胡凡磊,孙晓麟,李春,刘栩,叶华,邓立宗,李茹,栗占国. 类风湿关节炎临床分层及其特征的横断面研究[J]. 北京大学学报(医学版), 2022, 54(6): 1068-1073. |
| [9] | 刘蕊,赵金霞,闫良. 类风湿关节炎合并下肢静脉血栓患者的临床特点[J]. 北京大学学报(医学版), 2022, 54(6): 1079-1085. |
| [10] | 张警丰,金银姬,魏慧,姚中强,赵金霞. 类风湿关节炎患者生活质量与疾病活动度的横断面研究[J]. 北京大学学报(医学版), 2022, 54(6): 1086-1093. |
| [11] | 高超,陈立红,王莉,姚鸿,黄晓玮,贾语博,刘田. 类风湿关节炎合并纤维肌痛简易分类标准的临床验证[J]. 北京大学学报(医学版), 2022, 54(2): 278-282. |
| [12] | 娄雪,廖莉,李兴珺,王楠,刘爽,崔若玫,徐健. 类风湿关节炎患者外周血TWEAK基因启动子区甲基化状态及其表达[J]. 北京大学学报(医学版), 2021, 53(6): 1020-1025. |
| [13] | 钟华,徐丽玲,白明欣,苏茵. 类风湿关节炎患者趋化因子CXCL9和CXCL10在骨侵蚀中的作用[J]. 北京大学学报(医学版), 2021, 53(6): 1026-1031. |
| [14] | 罗靓,霍文岗,张钦,李春. 类风湿关节炎合并角膜溃疡的临床特点和相关因素分析[J]. 北京大学学报(医学版), 2021, 53(6): 1032-1036. |
| [15] | 张璐,胡小红,陈澄,蔡月明,王庆文,赵金霞. 类风湿关节炎初治患者颈椎失稳情况及临床特征[J]. 北京大学学报(医学版), 2021, 53(6): 1049-1054. |
|
||