北京大学学报(医学版) ›› 2023, Vol. 55 ›› Issue (2): 339-342. doi: 10.19723/j.issn.1671-167X.2023.02.020
程序性细胞死亡1-配体1在不同免疫组织化学染色方法的一致性比较
- 北京大学第一医院病理科,北京 100034
Consistency comparison of programmed cell death 1-ligand 1 in different immuno-histochemical staining methods
Dong LI,Ji-ting DI,Yan XIONG*( )
)
- Department of Pathology, Peking University First Hospital, Beijing 100034, China
摘要:
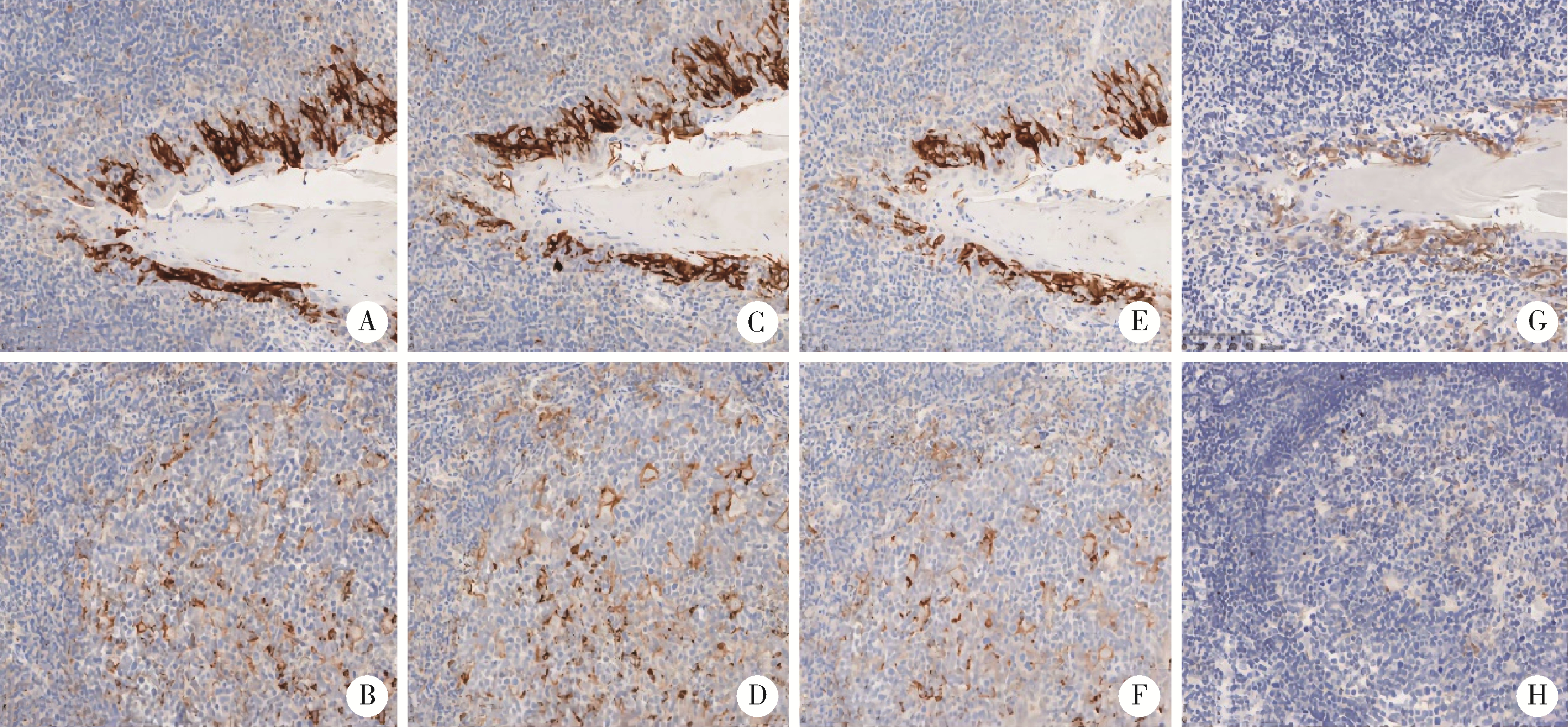
目的: 比较程序性细胞死亡1-配体1(programmed cell death 1-ligand 1, PD-L1,克隆号E1L3N、22C3、SP263)在不同免疫组织化学染色方法的一致性。方法: 第一步,筛选最优流程:采用PD-L1(克隆号E1L3N)抗体分别按推荐流程、自建流程①、自建流程②与自建流程③,对5例扁桃体组织完成平行免疫组织化学染色,专科病理医生对全部切片进行质量评分(0~6分),筛选出评分最高的流程;第二步,用最优流程与两种标准流程的评分结果进行一致性比较:选取近两年北京大学第一医院病理最终诊断为非小细胞肺癌的标本共32例,用筛选出的自建流程①、与SP263标准流程及22C3标准流程对32病例各自进行平行染色,全部染色片由专科病理医师行阳性肿瘤细胞比例评分(tumor proportion score,TPS)。评分结果按<1%,≥1%至<10%,≥10%至<50%,≥50%分组,分析PD-L1检测抗体克隆号E1L3N与22C3、SP263染色结果的一致性。结果: 扁桃体染色切片质量评分(0~6分)如下:推荐流程为5、5、5、5、5分;自建流程①为5、6、6、5、6分;自建流程②为4、4、4、4、4分;自建流程③为3、3、3、3、3分。自建流程①的结果总体评分最高,选用自建流程①完成32例非小细胞肺癌病例的免疫组组织化学染色,TPS评分为:自建流程①,<1%共6例,≥1%至<10%共5例,≥10%至 < 50%共10例,≥50%共11例;22C3标准流程,<1%共5例,≥1%至<10%共3例,≥10%至<50%共13例,≥50%共11例;SP263标准流程,<1%共7例,≥1%至<10%共4例,≥10%至<50%共11例,≥50%共10例。一致性检验结果为:自建流程①和22C3标准流程,κ值为0.736(P <0.001),一致性好;自建流程①和SP263标准流程,κ值为0.914(P <0.001),一致性极好。结论: 采用PD-L1(克隆号E1L3N)抗体,在罗氏Ventana Benchmark GX平台,通过自建染色流程①完成,可获得良好质量的染色切片;在非小细胞肺癌病例的检测中,自建染色流程①与22C3标准流程和SP263标准流程一致性好。
中图分类号:
- R365
| 1 |
Hu-Lieskovan S , Bhaumik S , Dhodapkar K , et al. SITC cancer immunotherapy resource document: A compass in the land of biomarker discovery[J]. J Immunother Cancer, 2020, 8 (2): e000705.
doi: 10.1136/jitc-2020-000705 |
| 2 |
Parra ER , Villalobos P , Mino B , et al. Comparison of different antibody clones for immunohistochemistry detection of programmed cell death ligand 1(PD-L1) on non small cell lung carcinoma[J]. Appl immunohistochem mol morphol, 2018, 26 (2): 83- 93.
doi: 10.1097/PAI.0000000000000531 |
| 3 |
Scorer P , Scott M , Lawson N , et al. Consistency of tumor and immune cell programmed cell death ligand-1 expression within and between tumor blocks using the VENTANA SP263 assay[J]. Diagn Pathol, 2018, 13 (1): 47.
doi: 10.1186/s13000-018-0725-9 |
| 4 |
Kim SY , Kim TE , Park CK , et al. Comprehensive comparison of 22C3 and SP263 PD-L1 expression in non small cell lung cancer using routine clinical and conditioned archives[J]. Cancers (Basel), 2022, 14 (13): 3138.
doi: 10.3390/cancers14133138 |
| 5 | Song L , Zeng L , Yan H , et al. Validation of E1L3N antibody for PD-L1 detection and prediction of pembrolizumab response in non small cell lung cancer[J]. Commun Med (Lond), 2022, 2 (1): 137. |
| 6 |
Lawson NL , Dix CI , Scorer PW , et al. Mapping the binding sites of antibodies utilized in programmed cell death ligand-1 predictive immunohistochemical assays for use with immuno-oncology therapies[J]. Mod Pathol, 2020, 33 (4): 518- 530.
doi: 10.1038/s41379-019-0372-z |
| 7 |
Keppens C , Dequeker EM , Pauwels P , et al. PD-L1 immunohistochemistry in non small cell lung cancer: Unraveling differences in staining concordance and interpretation[J]. Virchows Arch, 2021, 478 (5): 827- 839.
doi: 10.1007/s00428-020-02976-5 |
| 8 | Xu H , Dong X , Zhao H , et al. Clinical evaluation of a laboratory-developed test using clone E1L3N for the detection of PD-L1 expression status in non small cell lung cancer[J]. J Clin Lab Anal, 2021, 35 (3): e23696. |
| 9 |
Ilie M , Khambata-Ford S , Copie-Bergman C , et al. Use of the 22C3 anti-PD-L1 antibody to determine PD-L1 expression in multiple automated immunohistochemistry platforms[J]. PLoS One, 2017, 12 (8): e0183023.
doi: 10.1371/journal.pone.0183023 |
| 10 | Schalper KA , Daniel CH , Joseph M , et al. Clinical significance of PD-L1 protein expression on tumor-associated macrophages in lung cancer[J]. J Immunother Cancer, 2015, 3 (2): 415. |
| 11 |
McLaughlin J , Han G , Schalper KA , et al. Quantitative assessment of the heterogeneity of PD-L1 expression in non small cell lung cancer[J]. JAMA Oncol, 2016, 2 (1): 46- 54.
doi: 10.1001/jamaoncol.2015.3638 |
| [1] | 熊焰,张波,聂立功,吴世凯,赵虎,李东,邸吉廷. 胸部SMARCA4缺失性未分化肿瘤的病理诊断与联合免疫检测点抑制剂治疗[J]. 北京大学学报(医学版), 2023, 55(2): 351-356. |
| [2] | 朱巧,任翠,张艳,李美娇,王晓华. 能谱CT诊断非小细胞肺癌纵隔淋巴结转移的应用价值[J]. 北京大学学报(医学版), 2020, 52(4): 730-737. |
| [3] | 欧阳雨晴,倪莲芳,刘新民. 恶性孤立性肺结节患者预后因素分析[J]. 北京大学学报(医学版), 2020, 52(1): 158-162. |
| [4] | 张春凤,刘云,陆敏,杜晓娟. hUTP14a在非小细胞肺癌组织中的表达[J]. 北京大学学报(医学版), 2019, 51(1): 145-150. |
|
||