北京大学学报(医学版) ›› 2024, Vol. 56 ›› Issue (2): 284-292. doi: 10.19723/j.issn.1671-167X.2024.02.013
特发性炎性肌病完全临床应答相关因素的单中心真实世界研究
赖展鸿1,李嘉辰1,贠泽霖1,张永刚2,张昊3,邢晓燕1,邵苗1,金月波1,王乃迪1,李依敏4,李玉慧1,*( ),栗占国1,*(
),栗占国1,*( )
)
- 1. 北京大学人民医院风湿免疫科,北京 100044
2. 保定市第一中心医院风湿免疫科,河北保定 071000
3. 大连市中心医院风湿免疫科,辽宁大连 116089
4. 浙江大学医学院附属第一医院风湿免疫科,杭州 310003
A unicenter real-world study of the correlation factors for complete clinical response in idiopathic inflammatory myopathies
Zhanhong LAI1,Jiachen LI1,Zelin YUN1,Yonggang ZHANG2,Hao ZHANG3,Xiaoyan XING1,Miao SHAO1,Yuebo JIN1,Naidi WANG1,Yimin LI4,Yuhui LI1,*( ),Zhanguo LI1,*(
),Zhanguo LI1,*( )
)
- 1. Department of Rheumatology and Immunology, Peking University People's Hospital, Beijing 100044, China
2. Department of Rheumatology and Immunology, Baoding First Hospital, Baoding 071000, Hebei, China
3. Department of Rheumatology and Immunology, Dalian Municipal Central Hospital, Dalian 116089, Liaoning, China
4. Department of Rheumatology and Immunology, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, China
摘要:
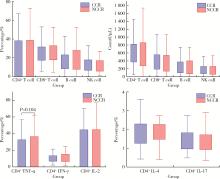
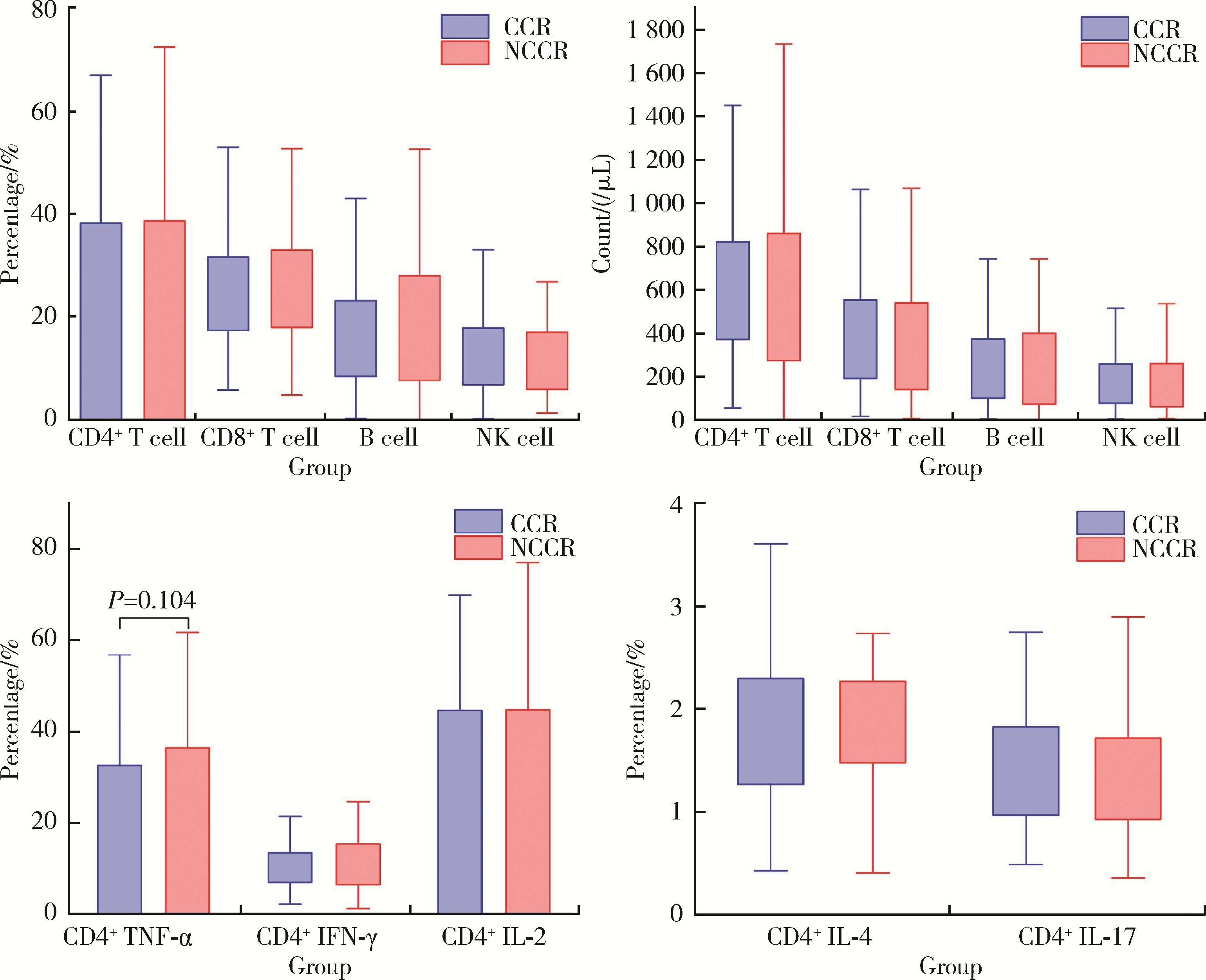
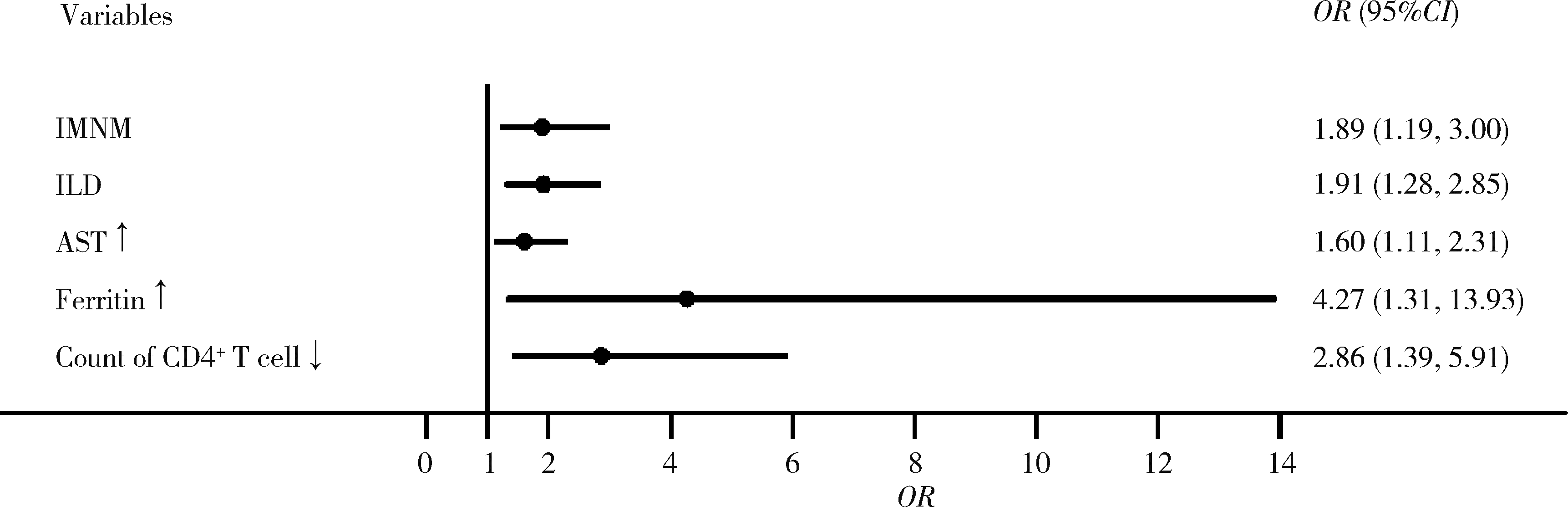
目的: 探究影响特发性炎性肌病(idiopathic inflammatory myopathies,IIMs)患者对常规治疗完全临床应答的相关因素。方法: 纳入2000年1月至2023年6月于北京大学人民医院就诊的IIMs患者,通过分析患者的临床特征、实验室检查、免疫学指标和治疗用药,明确影响患者对常规治疗完全临床应答的相关因素。结果: 共纳入635例IIMs患者,其中518例患者完成随访,平均随访时间36.8个月,总体完全临床应答率为50.0%(259/518)。各临床亚型中,皮肌炎(dermatomyositis,DM)、抗合成酶综合征(anti-synthetase syndrome,ASS)和免疫介导坏死性肌病(immune-mediated necrotizing myopathy,IMNM)的完全临床应答率分别为53.5%、48.9%和39.0%。未完全临床应答组与完全临床应答组相比,在临床特征方面,发热(P=0.002)和快速进展型间质性肺病(rapid progressive interstitial lung disease,RP-ILD)(P=0.014)的发生率较高;在实验室检查方面,谷草转氨酶、乳酸脱氢酶、D-二聚体、红细胞沉降率、C反应蛋白和血清铁蛋白水平较高;在治疗用药方面,激素和静脉注射免疫球蛋白(intravenous immunoglobin,IVIG)的使用比例均较高。IMNM(P=0.007)、间质性肺病(P=0.001)、谷草转氨酶高(P=0.012)、血清铁蛋白高(P=0.016)和外周血CD4+T细胞计数低(P=0.004)是IIMs未完全临床应答的危险因素。结论: IIMs患者的总体完全临床应答率低,IMNM亚型最低; 起病时存在间质性肺病、谷草转氨酶高、血清铁蛋白高或外周血CD4+T细胞计数低的患者应给予积极治疗。
中图分类号:
- R593.26
| 1 |
Tanboon J , Nishino I . Classification of idiopathic inflammatory myopathies: Pathology perspectives[J]. Curr Opin Neurol, 2019, 32 (5): 704- 714.
doi: 10.1097/WCO.0000000000000740 |
| 2 |
DeWane ME , Waldman R , Lu J . Dermatomyositis: Clinical features and pathogenesis[J]. J Am Acad Dermatol, 2020, 82 (2): 267- 281.
doi: 10.1016/j.jaad.2019.06.1309 |
| 3 | 罗澜, 邢晓燕, 肖云抒, 等. 抗合成酶综合征合并心脏受累患者的临床及免疫学特征[J]. 北京大学学报(医学版), 2021, 53 (6): 1078- 1082. |
| 4 |
Marco JL , Collins BF . Clinical manifestations and treatment of antisynthetase syndrome[J]. Best Pract Res Clin Rheumatol, 2020, 34 (4): 101503.
doi: 10.1016/j.berh.2020.101503 |
| 5 |
Pinal-Fernandez I , Casal-Dominguez M , Mammen AL . Immune-mediated necrotizing myopathy[J]. Curr Rheumatol Rep, 2018, 20 (4): 21.
doi: 10.1007/s11926-018-0732-6 |
| 6 |
Wolstencroft PW , Chung L , Li S , et al. Factors associated with clinical remission of skin disease in dermatomyositis[J]. JAMA Dermatol, 2018, 154 (1): 44- 51.
doi: 10.1001/jamadermatol.2017.3758 |
| 7 |
Lundberg IE , Fujimoto M , Vencovsky J , et al. Idiopathic inflammatory myopathies[J]. Nat Rev Dis Primers, 2021, 7 (1): 86.
doi: 10.1038/s41572-021-00321-x |
| 8 |
Bohan A , Peter JB . Polymyositis and dermatomyositis (first of two parts)[J]. N Engl J Med, 1975, 292 (7): 344- 347.
doi: 10.1056/NEJM197502132920706 |
| 9 |
Bohan A , Peter JB . Polymyositis and dermatomyositis (second of two parts)[J]. N Engl J Med, 1975, 292 (8): 403- 407.
doi: 10.1056/NEJM197502202920807 |
| 10 |
Lundberg IE , Tjärnlund A , Bottai M , et al. 2017 European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups[J]. Arthritis Rheumatol, 2017, 69 (12): 2271- 2282.
doi: 10.1002/art.40320 |
| 11 |
Allenbach Y , Mammen AL , Benveniste O , et al. 224th ENMC International Workshop: Clinico-sero-pathological classification of immune-mediated necrotizing myopathies Zandvoort, The Netherlands, 14-16 October 2016[J]. Neuromuscul Disord, 2018, 28 (1): 87- 99.
doi: 10.1016/j.nmd.2017.09.016 |
| 12 |
Oddis CV , Rider LG , Reed AM , et al. International consensus guidelines for trials of therapies in the idiopathic inflammatory myopathies[J]. Arthritis Rheum, 2005, 52 (9): 2607- 2615.
doi: 10.1002/art.21291 |
| 13 |
Aggarwal R , Rider LG , Ruperto N , et al. 2016 American College of Rheumatology/European League Against Rheumatism criteria for minimal, moderate, and major clinical response in adult dermatomyositis and polymyositis: An International Myositis Assessment and Clinical Studies Group/Paediatric Rheumatology International Trials Organisation Collaborative Initiative[J]. Ann Rheum Dis, 2017, 76 (5): 792- 801.
doi: 10.1136/annrheumdis-2017-211400 |
| 14 | Tiniakou E , Mecoli CA , Kelly W , et al. Anti-MDA5-positive dermatomyositis and remission in a single referral centre population[J]. Clin Exp Rheumatol, 2023, 41 (2): 309- 315. |
| 15 | Rider LG , Werth VP , Huber AM , et al. Measures of adult and juvenile dermatomyositis, polymyositis, and inclusion body myositis: Physician and patient/parent global activity, manual muscle testing (MMT), health assessment questionnaire (HAQ)/childhood health assessment questionnaire (C-HAQ), childhood myositis assessment scale (CMAS), myositis disease activity assessment tool (MDAAT), disease activity score (DAS), short form 36 (SF-36), child health questionnaire (CHQ), physician global damage, myositis damage index (MDI), quantitative muscle testing (QMT), myositis functional index-2 (FI-2), myositis activities profile (MAP), inclusion body myositis functional rating scale (IBMFRS), cutaneous dermatomyositis disease area and severity index (CDASI), cutaneous assessment tool (CAT), dermatomyositis skin severity index (DSSI), skindex, and dermatology life quality index (DLQI)[J]. Arthritis Care Res (Hoboken), 2011, 63 (Suppl 11): S118- S157. |
| 16 | Li Y , Li Y , Wu J , et al. Predictors of Poor Outcome of Anti-MDA5-associated rapidly progressive interstitial lung disease in a Chinese cohort with dermatomyositis[J]. J Immunol Res, 2020, 2020, 2024869. |
| 17 |
Sultan SM , Ioannou Y , Moss K , et al. Outcome in patients with idiopathic inflammatory myositis: Morbidity and mortality[J]. Rheumatology (Oxford), 2002, 41 (1): 22- 26.
doi: 10.1093/rheumatology/41.1.22 |
| 18 | Lee JS , Lee JE , Hong S , et al. Prognostic factors for steroid-free remission in patients with idiopathic inflammatory myopathies: Importance of anthropometric measurements[J]. Ther Adv Musculoskelet Dis, 2020, 12, 1759720x20936822. |
| 19 | Watanabe E , Gono T , Kuwana M , et al. Predictive factors for sustained remission with stratification by myositis-specific autoantibodies in adult polymyositis/dermatomyositis[J]. Rheumatology (Oxford), 2020, 59 (3): 586- 593. |
| 20 |
Mariampillai K , Granger B , Amelin D , et al. Development of a new classification system for idiopathic inflammatory myopathies based on clinical manifestations and myositis-specific autoanti-bodies[J]. JAMA Neurol, 2018, 75 (12): 1528- 1537.
doi: 10.1001/jamaneurol.2018.2598 |
| 21 |
Watanabe Y , Uruha A , Suzuki S , et al. Clinical features and prognosis in anti-SRP and anti-HMGCR necrotising myopathy[J]. J Neurol Neurosurg Psychiatry, 2016, 87 (10): 1038- 1044.
doi: 10.1136/jnnp-2016-313166 |
| 22 |
Merlonghi G , Antonini G , Garibaldi M . Immune-mediated necrotizing myopathy (IMNM): A myopathological challenge[J]. Autoimmun Rev, 2022, 21 (2): 102993.
doi: 10.1016/j.autrev.2021.102993 |
| 23 | 杨红霞, 田小兰, 江薇, 等. 免疫介导坏死性肌病的临床和病理特征分析[J]. 北京大学学报(医学版), 2019, 51 (6): 989- 995. |
| 24 | Tiniakou E , Pinal-Fernandez I , Lloyd TE , et al. More severe disease and slower recovery in younger patients with anti-3-hydro-xy-3-methylglutaryl-coenzyme A reductase-associated autoimmune myopathy[J]. Rheumatology (Oxford), 2017, 56 (5): 787- 794. |
| 25 |
Day JA , Limaye V . Immune-mediated necrotising myopathy: A critical review of current concepts[J]. Semin Arthritis Rheum, 2019, 49 (3): 420- 429.
doi: 10.1016/j.semarthrit.2019.04.002 |
| 26 |
Redondo-Benito A , Curran A , Villar-Gomez A , et al. Opportunistic infections in patients with idiopathic inflammatory myopathies[J]. Int J Rheum Dis, 2018, 21 (2): 487- 496.
doi: 10.1111/1756-185X.13255 |
| 27 |
Sun S , Chen Z , Zhang D , et al. Description and analysis of a novel subtype of the anti-synthetase syndrome characterized by frequent attacks of fever and systemic inflammation in a single-center cohort study[J]. Front Immunol, 2021, 12, 729602.
doi: 10.3389/fimmu.2021.729602 |
| 28 |
Debray MP , Borie R , Revel MP , et al. Interstitial lung disease in anti-synthetase syndrome: Initial and follow-up CT findings[J]. Eur J Radiol, 2015, 84 (3): 516- 523.
doi: 10.1016/j.ejrad.2014.11.026 |
| 29 |
Hallowell RW , Ascherman DP , Danoff SK . Pulmonary manifestations of polymyositis/dermatomyositis[J]. Semin Respir Crit Care Med, 2014, 35 (2): 239- 248.
doi: 10.1055/s-0034-1371528 |
| 30 |
李玉慧, 姚海红, 张学武, 等. 94例皮肌炎患者器官受累及免疫学特征分析[J]. 北京大学学报(医学版), 2010, 42 (2): 140- 142.
doi: 10.3969/j.issn.1671-167X.2010.02.005 |
| 31 |
Johnson C , Pinal-Fernandez I , Parikh R , et al. Assessment of mortality in autoimmune myositis with and without associated interstitial lung disease[J]. Lung, 2016, 194 (5): 733- 737.
doi: 10.1007/s00408-016-9896-x |
| 32 |
Chen IJ , Wu YJJ , Lin CW , et al. Interstitial lung disease in polymyositis and dermatomyositis[J]. Clin Rheumatol, 2009, 28 (6): 639- 646.
doi: 10.1007/s10067-009-1110-6 |
| 33 |
Hallowell RW , Danoff SK . Diagnosis and management of myositis-associated lung disease[J]. Chest, 2023, 163 (6): 1476- 1491.
doi: 10.1016/j.chest.2023.01.031 |
| 34 |
Li L , Wang H , Wang Q , et al. Myositis-specific autoantibodies in dermatomyositis/polymyositis with interstitial lung disease[J]. J Neurol Sci, 2019, 397, 123- 128.
doi: 10.1016/j.jns.2018.12.040 |
| 35 |
Srivastava P , Dwivedi S , Misra R . Myositis-specific and myositis-associated autoantibodies in Indian patients with inflammatory myositis[J]. Rheumatol Int, 2016, 36 (7): 935- 943.
doi: 10.1007/s00296-016-3494-3 |
| 36 | Li Y , Gao X , Li Y , et al. Predictors and mortality of rapidly progressive interstitial lung disease in patients with idiopathic inflammatory myopathy: A series of 474 patients[J]. Front Med (Lausanne), 2020, 7, 363. |
| 37 |
Jiang W , Shi JL , Yang HX , et al. Long-term outcomes and prognosis factors in patients with idiopathic inflammatory myopathies based on myositis-specific autoantibodies: A single cohort study[J]. Arthritis Care Res (Hoboken), 2023, 75 (5): 1175- 1182.
doi: 10.1002/acr.24993 |
| 38 | Komac A , Gokcen N , Yazici A , et al. The role of lactate dehydrogenase-to-albumin ratio in clinical evaluation of adult-onset Still's disease[J]. Int J Clin Pract, 2021, 75 (10): e14615. |
| 39 |
Bohan A , Peter JB , Bowman RL , et al. Computer-assisted analysis of 153 patients with polymyositis and dermatomyositis[J]. Medicine (Baltimore), 1977, 56 (4): 255- 286.
doi: 10.1097/00005792-197707000-00001 |
| 40 | Tymms KE , Webb J . Dermatopolymyositis and other connective tissue diseases: A review of 105 cases[J]. J Rheumatol, 1985, 12 (6): 1140- 1148. |
| 41 | Niu Q , Zhao LQ , Ma WL , et al. A new predictive model for the prognosis of MDA5(+) DM-ILD[J]. Front Med (Lausanne), 2022, 9, 908365. |
| 42 | Ding Y , Ge Y . Anti-melanoma differentiation-associated gene 5 antibody-positive dermatomyositis complicated with macrophage activation syndrome[J]. Ther Adv Chronic Dis, 2022, 13, 20406223221098128. |
| 43 |
Chen F , Wang D , Shu X , et al. Anti-MDA5 antibody is associa-ted with A/SIP and decreased T cells in peripheral blood and predicts poor prognosis of ILD in Chinese patients with dermatomyositis[J]. Rheumatol Int, 2012, 32 (12): 3909- 3915.
doi: 10.1007/s00296-011-2323-y |
| 44 | 朱冯赟智, 邢晓燕, 汤晓菲, 等. 肌炎合并血栓栓塞患者的临床及免疫学特征[J]. 北京大学学报(医学版), 2020, 52 (6): 995- 1000. |
| 45 |
Davalos D , Akassoglou K . Fibrinogen as a key regulator of inflammation in disease[J]. Semin Immunopathol, 2012, 34 (1): 43- 62.
doi: 10.1007/s00281-011-0290-8 |
| [1] | 李志存, 吴天俣, 梁磊, 范宇, 孟一森, 张骞. 穿刺活检单针阳性前列腺癌术后病理升级的危险因素分析及列线图模型构建[J]. 北京大学学报(医学版), 2024, 56(5): 896-901. |
| [2] | 颜野,李小龙,夏海缀,朱学华,张羽婷,张帆,刘可,刘承,马潞林. 前列腺癌根治术后远期膀胱过度活动症的危险因素[J]. 北京大学学报(医学版), 2024, 56(4): 589-593. |
| [3] | 陈延,李况蒙,洪锴,张树栋,程建星,郑仲杰,唐文豪,赵连明,张海涛,姜辉,林浩成. 阴茎海绵体注射试验对阴茎血管功能影响的回顾性研究[J]. 北京大学学报(医学版), 2024, 56(4): 680-686. |
| [4] | 庞博,郭桐君,陈曦,郭华棋,石嘉章,陈娟,王欣梅,李耀妍,单安琪,余恒意,黄婧,汤乃军,王艳,郭新彪,李国星,吴少伟. 天津与上海35岁以上人群氮氧化物个体暴露水平及其影响因素[J]. 北京大学学报(医学版), 2024, 56(4): 700-707. |
| [5] | 和静,房中则,杨颖,刘静,马文瑶,霍勇,高炜,武阳丰,谢高强. 血浆中脂质代谢分子与颈动脉粥样硬化斑块、传统心血管危险因素及膳食因素的关系[J]. 北京大学学报(医学版), 2024, 56(4): 722-728. |
| [6] | 李正芳,罗采南,武丽君,吴雪,孟新艳,陈晓梅,石亚妹,钟岩. 抗氨基甲酰化蛋白抗体在诊断类风湿关节炎中的应用价值[J]. 北京大学学报(医学版), 2024, 56(4): 729-734. |
| [7] | 蔡珊,张依航,陈子玥,刘云飞,党佳佳,师嫡,李佳欣,黄天彧,马军,宋逸. 北京市中小学生身体活动时间现状及影响因素的路径[J]. 北京大学学报(医学版), 2024, 56(3): 403-410. |
| [8] | 张祖洪,陈天娇,马军. 中小学生青春发动时相与心血管代谢危险因素的相关性[J]. 北京大学学报(医学版), 2024, 56(3): 418-423. |
| [9] | 林郁婷,王华丽,田宇,巩俐彤,常春. 北京市老年人认知功能的影响因素[J]. 北京大学学报(医学版), 2024, 56(3): 456-461. |
| [10] | 朱金荣,赵亚娜,黄巍,赵微微,王悦,王松,苏春燕. 感染新型冠状病毒的血液透析患者的临床特征[J]. 北京大学学报(医学版), 2024, 56(2): 267-272. |
| [11] | 司筱芊,赵秀娟,朱凤雪,王天兵. 创伤出血性休克后急性呼吸窘迫综合征的危险因素[J]. 北京大学学报(医学版), 2024, 56(2): 307-312. |
| [12] | 李洋洋,侯林,马紫君,黄山雅美,刘捷,曾超美,秦炯. 孕期因素与婴儿牛奶蛋白过敏的关系[J]. 北京大学学报(医学版), 2024, 56(1): 144-149. |
| [13] | 刘晓强,周寅. 牙种植同期植骨术围术期高血压的相关危险因素[J]. 北京大学学报(医学版), 2024, 56(1): 93-98. |
| [14] | 罗靓,李云,王红彦,相晓红,赵静,孙峰,张晓盈,贾汝琳,李春. 抗内皮细胞抗体检测在早期流产中的预测价值[J]. 北京大学学报(医学版), 2023, 55(6): 1039-1044. |
| [15] | 游芳凝,罗靓,刘香君,张学武,李春. 未分化结缔组织病患者的妊娠结局、疾病演变及其影响因素[J]. 北京大学学报(医学版), 2023, 55(6): 1045-1052. |
|
||