北京大学学报(医学版) ›› 2024, Vol. 56 ›› Issue (3): 505-511. doi: 10.19723/j.issn.1671-167X.2024.03.018
托法替布通过JAK/STAT3通路抑制肺成纤维细胞向肌成纤维细胞转化
何珊1,2,陈炘1,2,程琦1,朱灵江1,张培玉1,童淑婷1,薛静1,杜燕1,*( )
)
- 1. 浙江大学医学院附属第二医院风湿免疫科, 杭州 310009
2. 浙江大学医学院附属金华医院风湿免疫科, 浙江金华 321000
Tofacitinib inhibits the transformation of lung fibroblasts into myofibroblasts through JAK/STAT3 pathway
Shan HE1,2,Xin CHEN1,2,Qi CHENG1,Lingjiang ZHU1,Peiyu ZHANG1,Shuting TONG1,Jing XUE1,Yan DU1,*( )
)
- 1. Department of Rheumatology, the Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, 310009, China
2. Department of Rheumatology, the Affiliated Jinhua Hospital of Zhejiang University School of Medicine, Jinhua, 321000, Zhejiang, China
摘要:
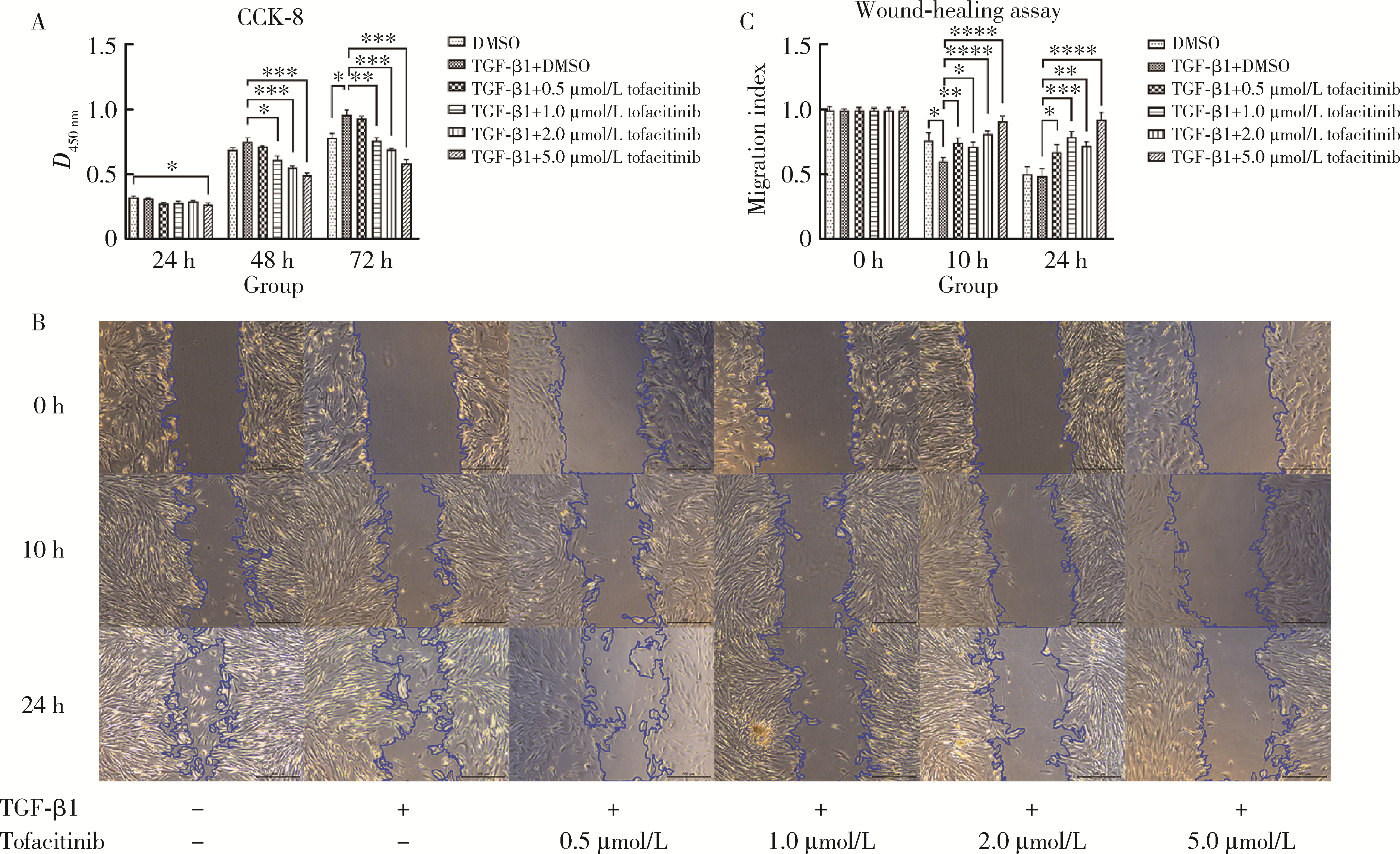
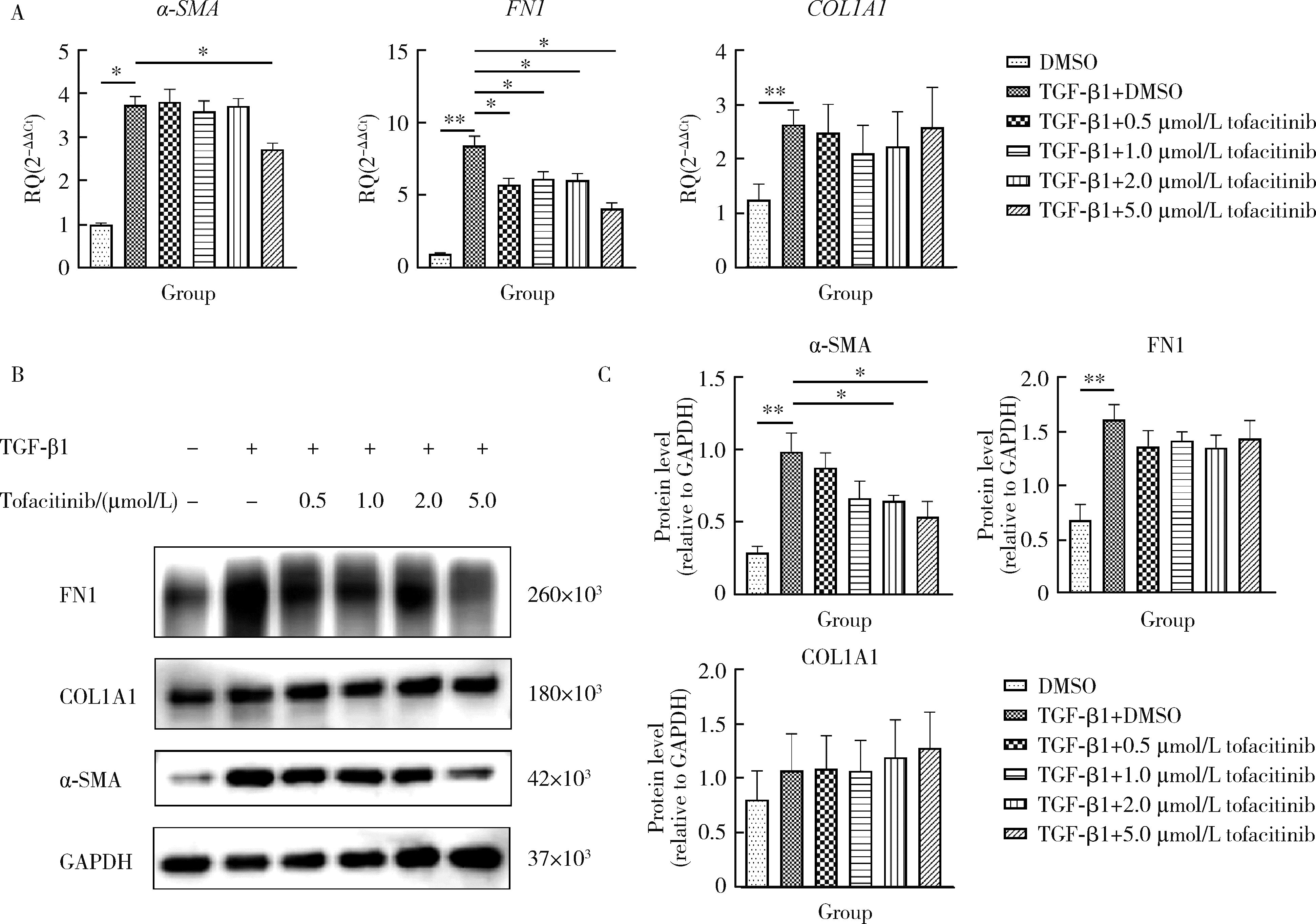
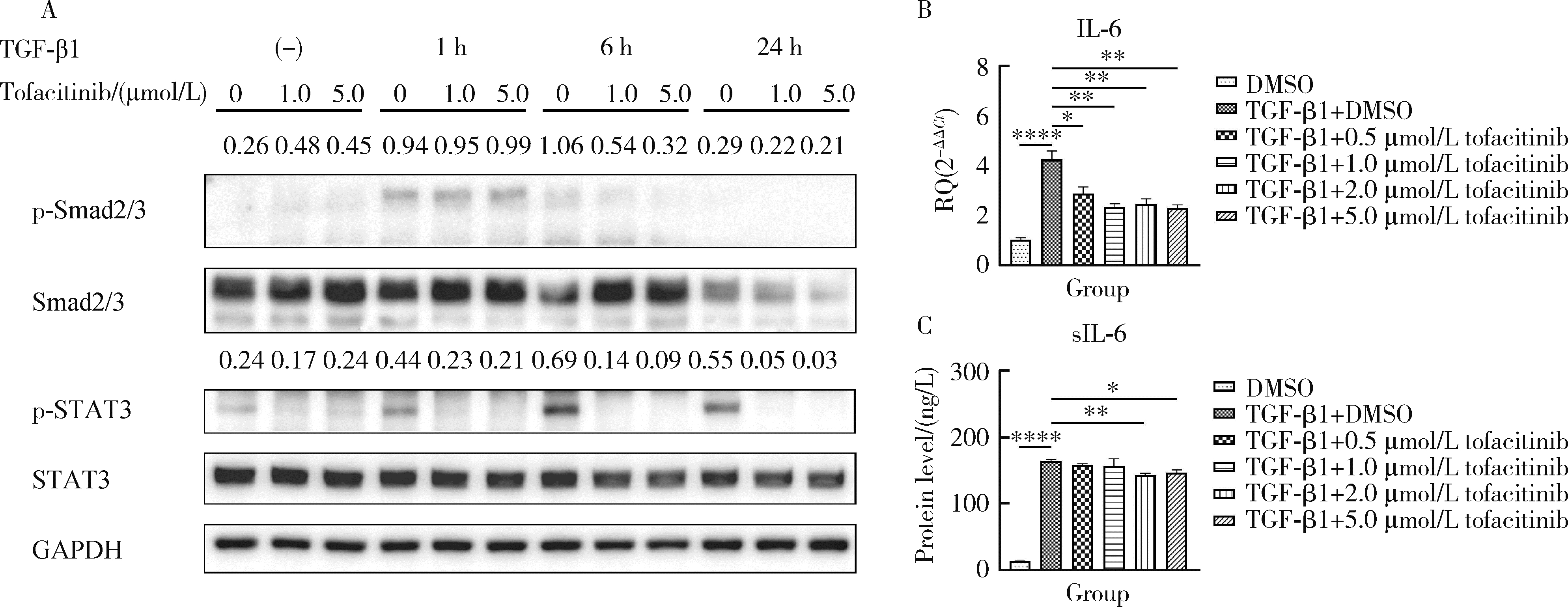
目的: 研究泛Janus激酶(Janus kinase, JAK)抑制剂托法替布(tofacitinib)对转化生长因子-β1(transforming growth factor-beta 1, TGF-β1)诱导的肺成纤维细胞向肌成纤维细胞转化的作用及机制,为临床治疗结缔组织病相关的间质性肺疾病提供理论依据。方法: (1) 体外培养人胚胎肺成纤维细胞(human fetal lung fibroblast 1, HFL-1),设立6个组,分别为DMSO空白对照组、TGF-β1诱导组、TGF-β1联合不同浓度托法替布(0.5、1.0、2.0、5.0 μmol/L)药物干预实验组。采用CCK-8法检测细胞活力,划痕愈合实验检测细胞迁移能力。(2)采用实时荧光定量PCR (quantitative real-time PCR,RT-PCR)、蛋白免疫印迹实验(Western blotting)检测α-平滑肌肌动蛋白(α-smooth muscle actin,α-SMA)、纤维连接蛋白(fibronectin,FN)、Ⅰ型胶原蛋白(collagen Ⅰ,COL1)基因及蛋白的表达水平。应用RT-PCR和酶联免疫吸附试验检测各组白细胞介素-6(interleukin-6,IL-6)的基因和细胞培养上清液中的蛋白水平。(3)在不同组的细胞培养基中加入DMSO载体对照,以1.0 μmol/L和5.0 μmol/L的托法替布预培养30 min,然后加入TGF-β1处理1 h、6 h和24 h。以Western blotting检测Smad2/3、信号传导和转录激活因子3(signal transducer and activator of transcription 3,STAT3)的蛋白磷酸化水平。结果: (1) 托法替布抑制TGF-β1诱导的HFL-1细胞活力及迁移能力。(2)与空白对照组相比,TGF-β1诱导组HFL-1的α-SMA、COL1A1和FN1基因表达显著上调(P < 0.05)。5.0 μmol/L托法替布干预组的α-SMA基因表达与TGF-β1诱导组相比显著下调(P < 0.05)。0.5~5.0 μmol/L托法替布各干预组与TGF-β1诱导组相比均可抑制FN1基因表达(P < 0.05)。各干预组与TGF-β1诱导组相比,COL1A1基因的表达无明显变化。(3)Western blotting结果提示,TGF-β1诱导组细胞α-SMA、FN1蛋白水平较对照组显著增加(P < 0.05),COL1A1表达未见明显差异。托法替布不同浓度干预组与TGF-β1诱导组相比,α-SMA蛋白表达均有下降,其中2.0 μmol/L和5.0 μmol/L干预组与诱导组间的差异有统计学意义(P < 0.05)。托法替布不同浓度干预组的FN1蛋白水平均有下降,但与TGF-β1诱导组相比差异无统计学意义。各干预组的COL1A1蛋白表达与TGF-β1诱导组相比差异无统计学意义。(4)TGF-β1诱导48 h,HFL-1细胞中IL-6基因及培养上清液中IL-6的表达水平较对照组显著升高,各浓度托法替布干预组与TGF-β1诱导组相比均有所降低。TGF-β1诱导1 h、6 h和24 h,STAT3蛋白磷酸化水平增加,托法替布预刺激抑制了6 h时Smad2/3的磷酸化水平,在1 h、6 h和24 h时均抑制了STAT3的磷酸化水平。结论: 托法替布能抑制TGF-β1诱导的HFL-1细胞向肌成纤维细胞方向转化,其机制可能是通过抑制Smad2/3经典通路以及抑制TGF-β1所诱导的STAT3磷酸化,从而对肺纤维化疾病进展发挥保护作用。
中图分类号:
- R563.13
| 1 |
Shao T , Shi X , Yang S , et al. Interstitial lung disease in connective tissue disease: A common lesion with heterogeneous mechanisms and treatment considerations[J]. Front Immunol, 2021, 12, 684699.
doi: 10.3389/fimmu.2021.684699 |
| 2 |
Cutolo M , Ruaro B , Montagna P , et al. Effects of selexipag and its active metabolite in contrasting the profibrotic myofibroblast activity in cultured scleroderma skin fibroblasts[J]. Arthritis Res Ther, 2018, 20 (1): 77.
doi: 10.1186/s13075-018-1577-0 |
| 3 | Truchetet ME , Brembilla NC , Chizzolini C . Current concepts on the pathogenesis of systemic sclerosis[J]. Clin Rev Allergy Immunol, 2023, 64 (3): 262- 283. |
| 4 |
Finnson KW , Almadani Y , Philip A . Non-canonical (non-SMAD2/3) TGF-β signaling in fibrosis: Mechanisms and targets[J]. Semin Cell Dev Biol, 2020, 101, 115- 122.
doi: 10.1016/j.semcdb.2019.11.013 |
| 5 |
You H , Xu D , Zhao J , et al. JAK inhibitors: Prospects in connective tissue diseases[J]. Clin Rev Allergy Immunol, 2020, 59 (3): 334- 351.
doi: 10.1007/s12016-020-08786-6 |
| 6 |
Wang W , Bhattacharyya S , Marangoni RG , et al. The JAK/STAT pathway is activated in systemic sclerosis and is effectively targeted by tofacitinib[J]. J Scleroderma Relat Disord, 2020, 5 (1): 40- 50.
doi: 10.1177/2397198319865367 |
| 7 |
Yoo H , Hino T , Hwang J , et al. Connective tissue disease-related interstitial lung disease (CTD-ILD) and interstitial lung abnorma-lity (ILA): Evolving concept of CT findings, pathology and management[J]. Eur J Radiol Open, 2022, 9, 100419.
doi: 10.1016/j.ejro.2022.100419 |
| 8 |
Montero P , Milara J , Roger I , et al. Role of JAK/STAT in interstitial lung diseases, molecular and cellular mechanisms[J]. Int J Mol Sci, 2021, 22 (12): 6211.
doi: 10.3390/ijms22126211 |
| 9 |
Lescoat A , Lelong M , Jeljeli M , et al. Combined anti-fibrotic and anti-inflammatory properties of JAK-inhibitors on macrophages in vitro and in vivo: Perspectives for scleroderma-associated interstitial lung disease[J]. Biochem Pharmacol, 2020, 178, 114103.
doi: 10.1016/j.bcp.2020.114103 |
| 10 |
Kagan P , Sultan M , Tachlytski I , et al. Both MAPK and STAT3 signal transduction pathways are necessary for IL-6-dependent hepatic stellate cells activation[J]. PLoS One, 2017, 12 (5): e0176173.
doi: 10.1371/journal.pone.0176173 |
| 11 | Chen W , Li Y , Hsu CT , et al. Connective tissue growth factor in hepatocytes is elevated by carbon tetrachloride via STAT3 activation[J]. Mol Med Rep, 2020, 21 (3): 1390- 1398. |
| 12 |
Dees C , Tomcik M , Palumbo-Zerr K , et al. JAK-2 as a novel mediator of the profibrotic effects of transforming growth factor β in systemic sclerosis[J]. Arthritis Rheum, 2012, 64 (9): 3006- 3015.
doi: 10.1002/art.34500 |
| 13 |
Chakraborty D , Šumová B , Mallano T , et al. Activation of STAT3 integrates common profibrotic pathways to promote fibroblast activation and tissue fibrosis[J]. Nat Commun, 2017, 8 (1): 1130.
doi: 10.1038/s41467-017-01236-6 |
| 14 |
Li HG , You PT , Xia Y , et al. Yu Gan Long ameliorates hepatic fibrosis by inhibiting PI3K/AKT, Ras/ERK and JAK1/STAT3 signaling pathways in CCl4-induced liver fibrosis rats[J]. Curr Med Sci, 2020, 40 (3): 539- 547.
doi: 10.1007/s11596-020-2211-3 |
| 15 |
Yan J , Zhang Z , Yang J , et al. JAK3/STAT6 stimulates bone marrow-derived fibroblast activation in renal fibrosis[J]. J Am Soc Nephrol, 2015, 26 (12): 3060- 3071.
doi: 10.1681/ASN.2014070717 |
| 16 |
Pedroza M , Le TT , Lewis K , et al. STAT-3 contributes to pulmonary fibrosis through epithelial injury and fibroblast-myofibroblast differentiation[J]. FASEB J, 2016, 30 (1): 129- 140.
doi: 10.1096/fj.15-273953 |
| 17 |
Levine RL , Pardanani A , Tefferi A , et al. Role of JAK2 in the pathogenesis and therapy of myeloproliferative disorders[J]. Nat Rev Cancer, 2007, 7 (9): 673- 683.
doi: 10.1038/nrc2210 |
| [1] | 邹雪,白小娟,张丽卿. 艾拉莫德联合托法替布治疗难治性中重度类风湿关节炎的疗效[J]. 北京大学学报(医学版), 2023, 55(6): 1013-1021. |
| [2] | 蔡天玉,朱振鹏,徐纯如,吉星,吕同德,郭振可,林健. 成纤维细胞生长因子受体2在肾透明细胞癌中的表达及意义[J]. 北京大学学报(医学版), 2022, 54(4): 628-635. |
| [3] | 吴燕芳,高飞,林滇恬,陈志涵,林禾. 托法替布联合治疗抗MDA5抗体阳性的无肌病皮肌炎并发快速进展型间质性肺病5例临床分析[J]. 北京大学学报(医学版), 2021, 53(5): 1012-1016. |
| [4] | 郜洪宇,孟焕新,侯建霞,黄宝鑫,李玮. 钙结合蛋白在健康牙周组织和实验性牙周炎组织的表达分布[J]. 北京大学学报(医学版), 2021, 53(4): 744-749. |
| [5] | 石冰清,袁晓静,赵玉鸣. 比较矿物三氧化物凝聚体及山东蜂胶乙醇提取物对牙髓成纤维细胞生物学性能的影响[J]. 北京大学学报(医学版), 2019, 51(6): 1108-1114. |
| [6] | 郑苗,詹凌璐,刘志强,李和平,谭建国. 不同等离子体处理氧化锆对人牙龈成纤维细胞黏附能力的影响[J]. 北京大学学报(医学版), 2019, 51(2): 315-320. |
| [7] | 李乾,常亮,苏冬梅,马旭. 粉防己碱对心肌成纤维细胞增殖、活化的影响[J]. 北京大学学报(医学版), 2018, 50(2): 331-334. |
| [8] | 贾双双,李伟阳,刘欣,李丽英. 转化生长因子-β1 通过产生活性氧诱导骨髓间充质干细胞分化为肌成纤维细胞[J]. 北京大学学报(医学版), 2015, 47(5): 737-742. |
| [9] | 杨竣, 王涛, 张岩, 徐华, 王少刚, 刘继红, 叶章群. 碱性成纤维细胞生长因子在老龄大鼠血清及阴茎海绵体中的含量[J]. 北京大学学报(医学版), 2015, 47(4): 582-585. |
| [10] | 雷玲 , 钟小宁, 赵铖, 米存东, 李佳荃, 曾晶晶. Th17细胞及相关细胞因子在系统性硬化病小鼠模型中的表达及意义[J]. 北京大学学报(医学版), 2012, 44(2): 259-264. |
| [11] | 战园, 王晓颖, 李盛林, 傅歆, 俞光岩, 曹彤, 刘鹤. 两种牙科材料对人胚胎干细胞来源成纤维细胞的细胞毒性研究[J]. 北京大学学报(医学版), 2012, 44(1): 1-5. |
| [12] | 刘畅, 宋振华, 秦泽莲. 人periostin干扰载体的构建及其对成纤维细胞目的基因表达的影响[J]. 北京大学学报(医学版), 2010, 42(5): 503-508. |
| [13] | 孙宇, 刘毅强, 冯国双, 李吉友. 转化生长因子β1在萎缩性胃炎发生中的作用[J]. 北京大学学报(医学版), 2009, 41(6): 635-639. |
| [14] | 李茹, 李霞, 张晓苹, 相晓红, 栗占国. 类风湿关节炎合并肺间质纤维化的临床特点[J]. 北京大学学报(医学版), 2009, 41(6): 674-677. |
| [15] | 林箐, 倪莲芳, 任雅丽, 刘新民. PPARγ和NF-κB在肺纤维化中的表达与意义[J]. 北京大学学报(医学版), 2009, 41(5): 545-547. |
|
||