北京大学学报(医学版) ›› 2025, Vol. 57 ›› Issue (3): 487-495. doi: 10.19723/j.issn.1671-167X.2025.03.012
代谢相关脂肪性肝病及其心脏代谢风险指标异常与不良妊娠结局的相关性
杨树涵1, 李奕昕2, 崔浩亮3, 王佑新1, 吴玉莹1, 王明月1, 杨依凡1, 恩卡尔·努尔1, 杨磊4,*( ), 王辉1,*(
), 王辉1,*( )
)
- 1. 北京大学公共卫生学院妇幼卫生学系, 北京 100191
2. 北京大学公共卫生学院流行病与卫生统计学系, 北京 100191
3. 北京大学公共卫生学院全球卫生学系, 北京 100191
4. 首都医科大学附属北京友谊医院妇产科, 北京 100050
Associations of metabolic dysfunction-associated steatotic liver disease and cardiometabolic risk factor abnormalities with adverse pregnancy outcomes
Shuhan YANG1, Yixin LI2, Haoliang CUI3, Youxin WANG1, Yuying WU1, Mingyue WANG1, Yifan YANG1, Nur Enkar1, Lei YANG4,*( ), Hui WANG1,*(
), Hui WANG1,*( )
)
- 1. Department of Maternal and Child Health, School of Public Health, Peking University, Beijing 100191, China
2. Department of Epidemiology and Biostatistics, School of Public Health, Peking University, Beijing 100191, China
3. Department of Global Health, School of Public Health, Peking University, Beijing 100191, China
4. Department of Obstetrics and Gynecology, Beijing Friendship Hospital, Capital University of Medical Sciences, Beijing 100050, China
摘要:
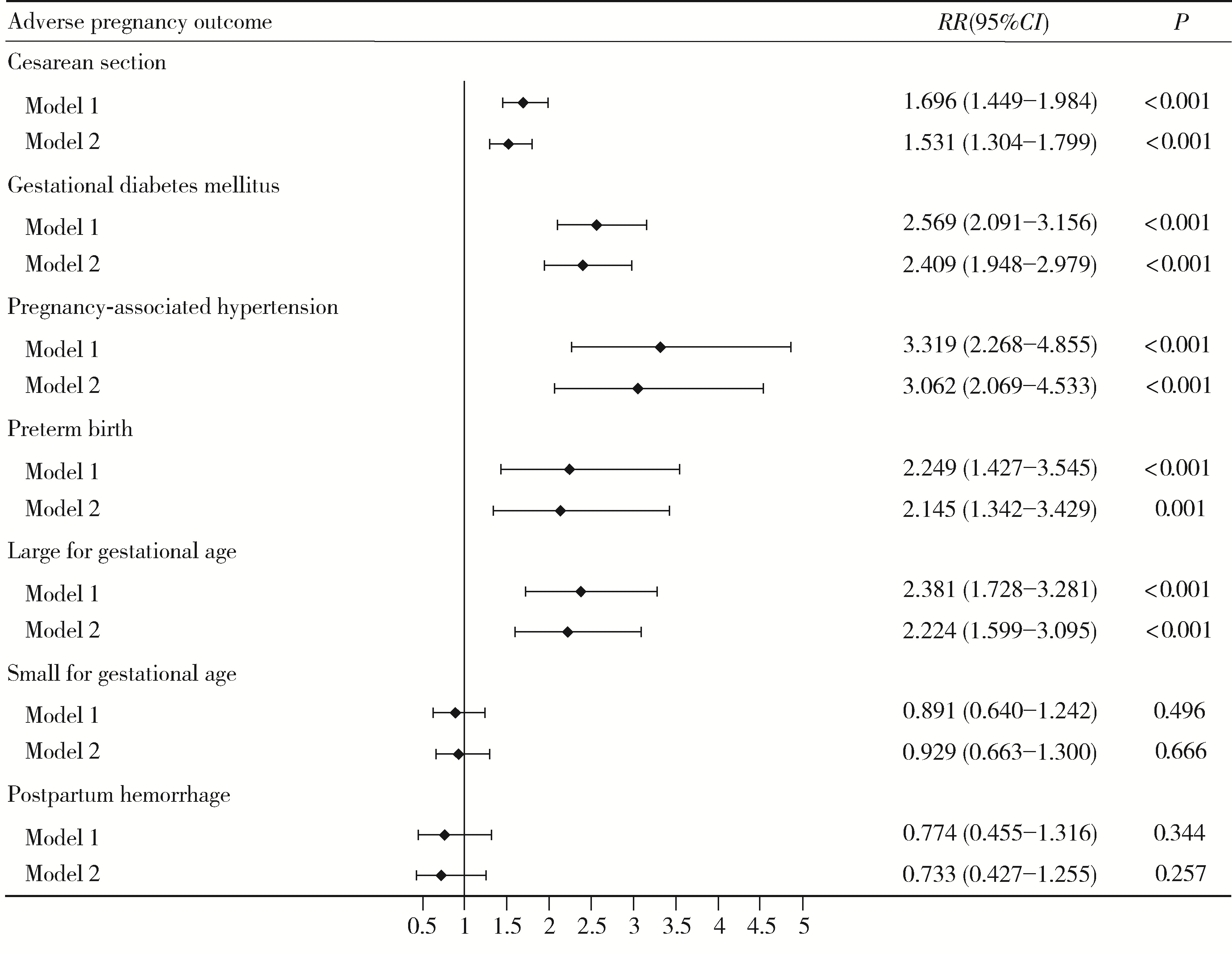
目的: 探讨代谢相关脂肪性肝病(metabolic dysfunction-associated steatotic liver disease, MASLD)与不良妊娠结局的相关性, 并分析心脏代谢风险指标(cardiometabolic risk factor, CMRF)异常的种类及其严重程度对该相关性的影响。方法: 采用回顾性队列研究, 选择2020年3月10日至2022年12月31日在首都医科大学附属北京友谊医院产科建档的单胎初产孕妇, 共纳入2 623名孕妇。记录基本信息和分娩结局, 并进行肝脏超声检查及相关产前检查, 对不良妊娠结局进行诊断。采用修正Poisson回归方法分析MASLD及其不同CMRF异常种类与不良妊娠结局的相关性, 并探究MASLD的CMRF异常程度变化与不良妊娠结局发生风险的相关性。结果: 调整年龄、孕期增重水平和受教育水平等协变量后, MASLD与剖宫产(RR=1.531, 95%CI:1.304~1.799, P < 0.001)、妊娠期糖尿病(RR=2.409, 95%CI:1.948~2.979, P < 0.001)、妊娠相关高血压(RR=3.062, 95%CI: 2.069~4.533, P < 0.001)、早产(RR=2.145, 95%CI: 1.342~3.429, P=0.001)和大于胎龄儿(RR=2.224, 95%CI: 1.599~3.095, P < 0.001)的风险升高相关。在控制其他种类CMRF异常的影响后, 不同CMRF异常MASLD孕妇的不良妊娠结局风险存在差异, 体重指数异常组的剖宫产、妊娠期糖尿病、妊娠相关高血压、早产和大于胎龄儿风险升高;血糖异常组的妊娠期糖尿病风险升高;血压异常组的妊娠相关高血压风险升高;高密度脂蛋白胆固醇异常组的剖宫产、妊娠期糖尿病和妊娠相关高血压风险升高;甘油三酯异常组的妊娠期糖尿病和早产风险升高。随着CMRF异常程度的加重, MASLD孕妇的剖宫产、妊娠期糖尿病、妊娠相关高血压、早产和大于胎龄儿风险持续升高(P均 < 0.05)。结论: 妊娠期MASLD与多种不良妊娠结局风险升高相关, 且MASLD的CMRF异常种类及严重程度对该相关性存在影响, 这提示在诊断MASLD时关注CMRF异常的具体情况, 有助于实施针对性干预并降低不良妊娠结局的发生风险。
中图分类号:
- R714.25
| 1 |
doi: 10.1111/jgh.16554 |
| 2 |
doi: 10.1016/j.jhep.2020.03.049 |
| 3 |
窦紫岩, 钱文红, 孔邻润, 等. 非酒精性脂肪性肝病检出率现状及其影响因素: 基于北京市32万人群数据[J]. 中国全科医学, 2024, 27 (2): 144-149, 155.
|
| 4 |
doi: 10.1056/NEJMra1011035 |
| 5 |
doi: 10.1161/HYPERTENSIONAHA.115.06667 |
| 6 |
doi: 10.1016/j.jhep.2016.05.013 |
| 7 |
中华医学会肝病学分会. 代谢相关(非酒精性)脂肪性肝病防治指南(2024年版)[J]. 中华肝脏病杂志, 2024, 32 (5): 418- 434.
|
| 8 |
doi: 10.1097/HEP.0000000000000520 |
| 9 |
doi: 10.1097/MEG.0000000000002802 |
| 10 |
|
| 11 |
|
| 12 |
doi: 10.1038/s41440-023-01354-3 |
| 13 |
中华人民共和国国家卫生和计划生育委员会. 成人体重判定[S]. 北京: 中国标准出版社, 2013.
|
| 14 |
中华人民共和国国家卫生健康委员会. 妊娠期妇女体重增长推荐值标准[S]. 北京: 中国标准出版社, 2022.
|
| 15 |
中华医学会肝病学分会脂肪肝和酒精性肝病学组, 中国医师协会脂肪性肝病专家委员会. 非酒精性脂肪性肝病防治指南(2018更新版)[J]. 中华肝脏病杂志, 2018, 26 (3): 195- 203.
|
| 16 |
doi: 10.2337/dc09-1848 |
| 17 |
Hypertension in pregnancy: Executive summary [J]. Obstet Gynecol, 2013, 122(5): 1122-1131.
|
| 18 |
doi: 10.1097/AOG.0000000000001711 |
| 19 |
doi: 10.1111/j.1651-2227.1996.tb14164.x |
| 20 |
中华人民共和国国家卫生健康委员会. 不同胎龄新生儿出生时生长评价标准[S]. 北京: 中国标准出版社, 2022.
|
| 21 |
谢幸, 孔北华, 段涛, 等. 妇产科学[M]. 9版 北京: 人民卫生出版社, 2018: 204.
|
| 22 |
doi: 10.1210/clinem/dgac567 |
| 23 |
|
| 24 |
doi: 10.1186/s12884-024-06556-2 |
| 25 |
doi: 10.1016/j.wombi.2019.06.011 |
| 26 |
|
| 27 |
doi: 10.1038/ncomms13496 |
| 28 |
doi: 10.3390/ijms24087465 |
| 29 |
doi: 10.1016/j.jacc.2010.05.034 |
| 30 |
doi: 10.1373/clinchem.2019.303990 |
| 31 |
doi: 10.1007/s00467-020-04579-3 |
| 32 |
doi: 10.1038/s41366-023-01299-0 |
| 33 |
doi: 10.3390/ijerph191710846 |
| [1] | 王文琼, 侯玉珂, 李春, 张学武. 系统性红斑狼疮患者不良妊娠结局的预测因素[J]. 北京大学学报(医学版), 2025, 57(3): 599-603. |
| [2] | 卞雯, 周文君, 吴天晨, 朱培静, 陈一诺, 原鹏波, 王学举, 王颖, 魏瑗, 赵扬玉. 单绒毛膜双羊膜囊双胎妊娠双胎之一胎死宫内对妊娠结局的影响[J]. 北京大学学报(医学版), 2025, 57(3): 592-598. |
| [3] | 马会超,李军,王永清. 妊娠合并炎症性肠病的临床特点[J]. 北京大学学报(医学版), 2024, 56(2): 260-266. |
| [4] | 游芳凝,罗靓,刘香君,张学武,李春. 未分化结缔组织病患者的妊娠结局、疾病演变及其影响因素[J]. 北京大学学报(医学版), 2023, 55(6): 1045-1052. |
| [5] | 孙希雅,陈艺璐,曾琳,闫丽盈,乔杰,李蓉,智旭. 不孕女性维生素D水平与抗苗勒氏管激素的相关性及对妊娠结局的预测[J]. 北京大学学报(医学版), 2023, 55(1): 167-173. |
| [6] | 郑晓娟, 邓晓莉, 刘湘源. 54例抗磷脂综合征患者的妊娠结局[J]. 北京大学学报(医学版), 2014, 46(2): 323-328. |
| [7] | 任昀,杨硕,杨蕊,李蓉,陈新娜,王海燕,马彩虹,刘平,乔杰. 促性腺激素释放激素激动剂长方案与拮抗剂方案对体外受精治疗妊娠结局的影响[J]. 北京大学学报(医学版), 2013, 45(6): 877-881. |
| [8] | 陈倩, 郭燕燕. 妊娠合并慢性肾炎19例临床分析及随访[J]. 北京大学学报(医学版), 2002, 34(1): 93-94. |
|
||