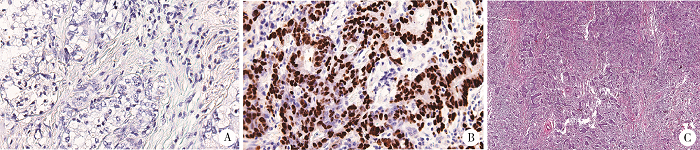
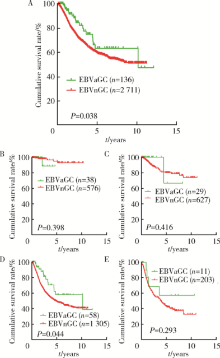
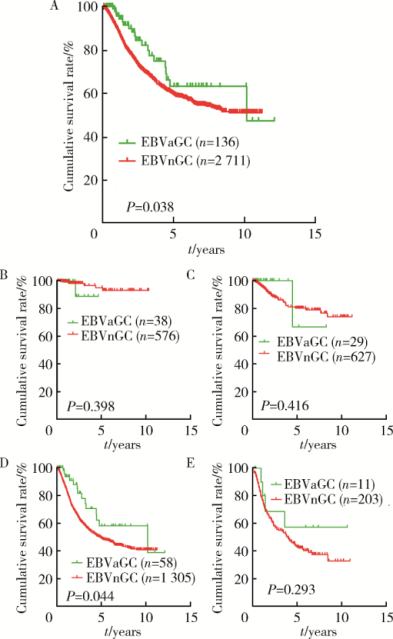
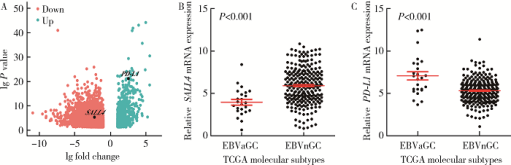
Journal of Peking University(Health Sciences) ›› 2019, Vol. 51 ›› Issue (3): 451-458. doi: 10.19723/j.issn.1671-167X.2019.03.012
Previous Articles Next Articles
Clinicopathological and molecular characteristics of Epstein-Barr virus associated gastric cancer: a single center large sample case investigation
Yang YANG1,Yi-qiang LIU2,Xiao-hong WANG3,Ke JI1,Zhong-wu LI2,Jian BAI4,Ai-rong YANG4,Ying HU3,Hai-bo HAN3,Zi-yu LI1,Zhao-de BU1,Xiao-jiang WU1,Lian-hai ZHANG1△( ),Jia-fu JI1△(
),Jia-fu JI1△( )
)
- 1. Department of Gastrointestinal Cancer Center
2. Department of Pathology
3. Department of Biobank, Key Laboratory of Carcinogenesis and Translational Research, Ministry of Education; Laboratory of Genetics, Peking University Cancer Hospital & Institute, Beijing 100142, China
4. Berry Oncology Corporation, Beijing 102206, China
CLC Number:
- R735.2
| [1] |
Horiuchi K, Mishima K, Ohsawa M , et al. Carcinoma of stomach and breast with lymphoid stroma: localisation of Epstein-Barr virus[J]. J Clin Pathol, 1994,47(6):538-540.
doi: 10.1136/jcp.47.6.538 |
| [2] |
Network CGAR . Comprehensive molecular characterization of gastric adenocarcinoma[J]. Nature, 2014,513(7517):202.
doi: 10.1038/nature13480 |
| [3] |
Baek DW, Kang BW, Hwang S , et al. Clinical significance of p53 protein expression, beta-catenin expression and HER2 expression for Epstein-Barr virus-associated gastric cancer[J]. Chonnam Med J, 2017,53(2):140-146.
doi: 10.4068/cmj.2017.53.2.140 |
| [4] |
Camargo MC, Kim WH, Chiaravalli AM , et al. Improved survival of gastric cancer with tumour Epstein-Barr virus positivity: an international pooled analysis[J]. Gut, 2014,63(2):236-243.
doi: 10.1136/gutjnl-2013-304531 |
| [5] |
Camargo M, Murphy G, Koriyama C , et al. Determinants of Epstein-Barr virus-positive gastric cancer: an international pooled analysis[J]. Brit J Cancer, 2011,105(1):38.
doi: 10.1038/bjc.2011.215 |
| [6] |
Murphy G, Pfeiffer R, Camargo MC , et al. Meta-analysis shows that prevalence of Epstein-Barr virus-positive gastric cancer differs based on sex and anatomic location[J]. Gastroenterology, 2009,137(3):824-833.
doi: 10.1053/j.gastro.2009.05.001 |
| [7] |
Dong M, Wang HY, Zhao XX , et al. Expression and prognostic roles of PIK3CA, JAK2, PD-L1, and PD-L2 in Epstein-Barr virus-associated gastric carcinoma[J]. Hum Pathol, 2016,53:25-34.
doi: 10.1016/j.humpath.2016.02.007 |
| [8] | Myers RB, Kudlow JE, Grizzle WE . Expression of transforming growth factor-alpha, epidermal growth factor and the epidermal growth factor receptor in adenocarcinoma of the prostate and benign prostatic hyperplasia[J]. Mod Pathol, 1993,6(6):733-737. |
| [9] |
Yong KJ, Gao C, Lim JS , et al. Oncofetal gene SALL4 in aggressive hepatocellular carcinoma[J]. N Engl J Med, 2013,368(24):2266-2276.
doi: 10.1056/NEJMoa1300297 |
| [10] |
Harn HJ, Chang JY, Wang MW , et al. Epstein-Barr virus-asso-ciated gastric adenocarcinoma in Taiwan[J]. Hum Pathol, 1995,26(3):267-271.
doi: 10.1016/0046-8177(95)90056-X |
| [11] |
Qiu K, Tomita Y, Hashimoto M , et al. Epstein-Barr virus in gastric carcinoma in Suzhou, China and Osaka, Japan: Association with clinico-pathologic factors and HLA-subtype[J]. Int J Can-cer, 1997,71(2):155-158.
doi: 10.1002/(ISSN)1097-0215 |
| [12] |
Liu S, Zhao Z, Han L , et al. Epstein-Barr virus infection in gastric remnant carcinoma and recurrent gastric carcinoma in Qingdao of Northern China[J]. PLoS One, 2016,11(2):e0148342.
doi: 10.1371/journal.pone.0148342 |
| [13] |
Sukawa Y, Yamamoto H, Nosho K , et al. Alterations in the human epidermal growth factor receptor 2-phosphatidylinositol 3-kinase-v-Akt pathway in gastric cancer[J]. World J Gastroenterol, 2012,18(45):6577-6586.
doi: 10.3748/wjg.v18.i45.6577 |
| [14] |
Kaizaki Y, Hosokawa O, Sakurai S , et al. Epstein-Barr virus-associated gastric carcinoma in the remnant stomach: de novo and metachronous gastric remnant carcinoma[J]. J Gastroenterol, 2005,40(6):570-577.
doi: 10.1007/s00535-005-1590-3 |
| [15] |
Zhang L, Xu Z, Xu X , et al. SALL4, a novel marker for human gastric carcinogenesis and metastasis[J]. Oncogene, 2014,33(48):5491-5500.
doi: 10.1038/onc.2013.495 |
| [16] |
Kim ST, Cristescu R, Bass AJ , et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metasta-tic gastric cancer[J]. Nat Med, 2018,24(9):1449-1458.
doi: 10.1038/s41591-018-0101-z |
| [1] | Junyong OU,Kunming NI,Lulin MA,Guoliang WANG,Ye YAN,Bin YANG,Gengwu LI,Haodong SONG,Min LU,Jianfei YE,Shudong ZHANG. Prognostic factors of patients with muscle invasive bladder cancer with intermediate-to-high risk prostate cancer [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 582-588. |
| [2] | Shuai LIU,Lei LIU,Zhuo LIU,Fan ZHANG,Lulin MA,Xiaojun TIAN,Xiaofei HOU,Guoliang WANG,Lei ZHAO,Shudong ZHANG. Clinical treatment and prognosis of adrenocortical carcinoma with venous tumor thrombus [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 624-630. |
| [3] | Le YU,Shaohui DENG,Fan ZHANG,Ye YAN,Jianfei YE,Shudong ZHANG. Clinicopathological characteristics and prognosis of multilocular cystic renal neoplasm of low malignant potential [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 661-666. |
| [4] | Zezhen ZHOU,Shaohui DENG,Ye YAN,Fan ZHANG,Yichang HAO,Liyuan GE,Hongxian ZHANG,Guoliang WANG,Shudong ZHANG. Predicting the 3-year tumor-specific survival in patients with T3a non-metastatic renal cell carcinoma [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 673-679. |
| [5] | Yangyi FANG,Qiang LI,Zhigao HUANG,Min LU,Kai HONG,Shudong ZHANG. Well-differentiated papillary mesothelial tumour of the tunica vaginalis: A case report [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 741-744. |
| [6] | Yuanyuan ZENG,Yun XIE,Daonan CHEN,Ruilan WANG. Related factors of euthyroid sick syndrome in patients with sepsis [J]. Journal of Peking University (Health Sciences), 2024, 56(3): 526-532. |
| [7] | Jian-bin LI,Meng-na LYU,Qiang CHI,Yi-lin PENG,Peng-cheng LIU,Rui WU. Early prediction of severe COVID-19 in patients with Sjögren’s syndrome [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 1007-1012. |
| [8] | Huan-rui LIU,Xiang PENG,Sen-lin LI,Xin GOU. Risk modeling based on HER-2 related genes for bladder cancer survival prognosis assessment [J]. Journal of Peking University (Health Sciences), 2023, 55(5): 793-801. |
| [9] | Zi-xuan XUE,Shi-ying TANG,Min QIU,Cheng LIU,Xiao-jun TIAN,Min LU,Jing-han DONG,Lu-lin MA,Shu-dong ZHANG. Clinicopathologic features and prognosis of young renal tumors with tumor thrombus [J]. Journal of Peking University (Health Sciences), 2023, 55(5): 802-811. |
| [10] | Han LU,Jian-yun ZHANG,Rong YANG,Le XU,Qing-xiang LI,Yu-xing GUO,Chuan-bin GUO. Clinical factors affecting the prognosis of lower gingival squamous cell carcinoma [J]. Journal of Peking University (Health Sciences), 2023, 55(4): 702-707. |
| [11] | Yun-fei SHI,Hao-jie WANG,Wei-ping LIU,Lan MI,Meng-ping LONG,Yan-fei LIU,Yu-mei LAI,Li-xin ZHOU,Xin-ting DIAO,Xiang-hong LI. Analysis of clinicopathological and molecular abnormalities of angioimmunoblastic T-cell lymphoma [J]. Journal of Peking University (Health Sciences), 2023, 55(3): 521-529. |
| [12] | Xiao-juan ZHU,Hong ZHANG,Shuang ZHANG,Dong LI,Xin LI,Ling XU,Ting LI. Clinicopathological features and prognosis of breast cancer with human epidermal growth factor receptor 2 low expression [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 243-253. |
| [13] | Yu-mei LAI,Zhong-wu LI,Huan LI,Yan WU,Yun-fei SHI,Li-xin ZHOU,Yu-tong LOU,Chuan-liang CUI. Clinicopathological features and prognosis of anorectal melanoma: A report of 68 cases [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 262-269. |
| [14] | Qi SHEN,Yi-xiao LIU,Qun HE. Mucinous tubular and spindle cell carcinoma of kidney: Clinicopathology and prognosis [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 276-282. |
| [15] | Wei-hua HOU,Shu-jie SONG,Zhong-yue SHI,Mu-lan JIN. Clinicopathological features of Helicobacter pylori-negative early gastric cancer [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 292-298. |
| Viewed | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
Full text 514
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
Abstract 995
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||
Cited |
|
|||||||||||||||||||||||||||||||||||||||||||||||||
| Shared | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Discussed | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
||