Journal of Peking University (Health Sciences) ›› 2021, Vol. 53 ›› Issue (2): 364-370. doi: 10.19723/j.issn.1671-167X.2021.02.022
Previous Articles Next Articles
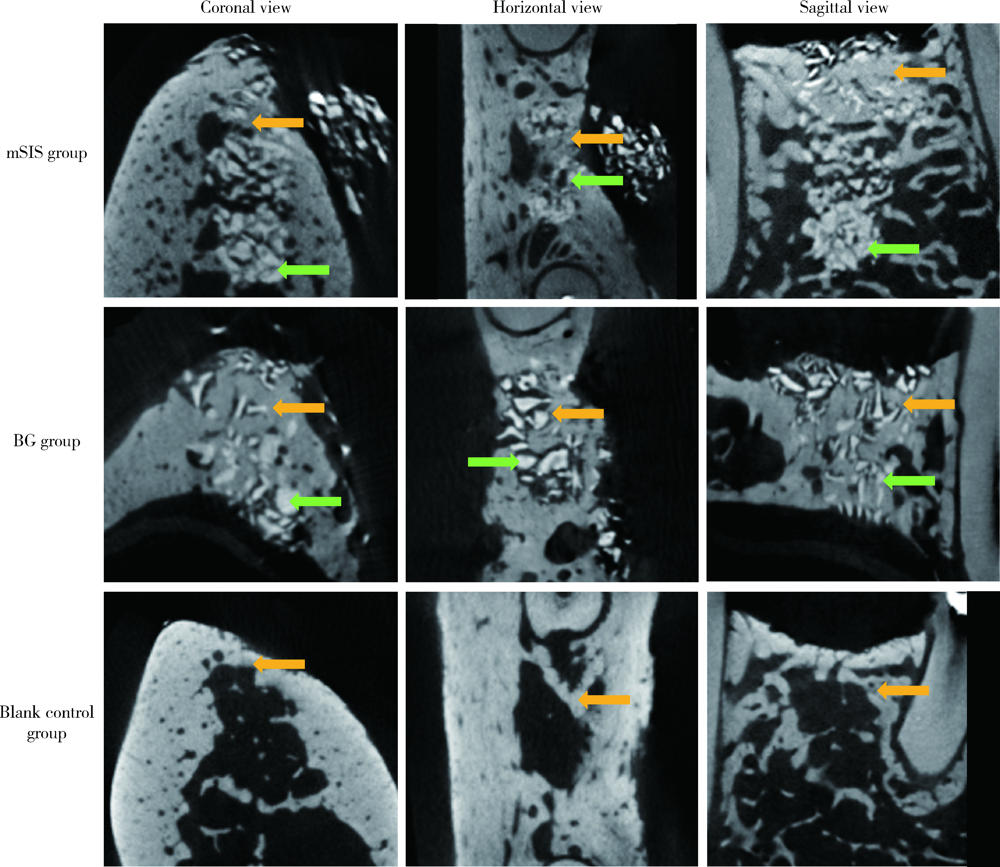
Efficacy of two barrier membranes and deproteinized bovine bone mineral on bone regeneration in extraction sockets: A microcomputed tomographic study in dogs
WANG Si-wen,YOU Peng-yue,LIU Yu-hua( ),WANG Xin-zhi,TANG Lin,WANG Mei
),WANG Xin-zhi,TANG Lin,WANG Mei
- Department of Prosthodontics, Peking University School and Hospital of Stomatology & National Clinical Research Center for Oral Diseases & National Engineering Laboratory for Digital and Material Technology of Stomatology & Beijing Key Laboratory of Digital Stomatology, Beijing 100081, China
CLC Number:
- R782.1
| [1] |
Ersanli S, Olgac V, Leblebicioglu B. Histologic analysis of alveolar bone following guided bone regeneration[J]. J Periodontol, 2004,75(5):750-756.
doi: 10.1902/jop.2004.75.5.750 pmid: 15212358 |
| [2] | Chiapasco M, Zaniboni M. Clinical outcomes of GBR procedures to correct peri-implant dehiscences and fenestrations: a systematic review[J]. Clin Oral Implants Res, 2009,20(Suppl 4):113-123. |
| [3] |
Oikarinen KS, Sandor GK, Kainulainen VT, et al. Augmentation of the narrow traumatized anterior alveolar ridge to facilitate dental implant placement[J]. Dent Traumatol, 2003,19(1):19-29.
pmid: 12656851 |
| [4] |
Amler MH. The time sequence of tissue regeneration in human extraction wounds[J]. Oral Surg Oral Med Oral Pathol, 1969,27(3):309-318.
pmid: 5251474 |
| [5] |
Nyman S, Lang NP, Buser D, et al. Bone regeneration adjacent to titanium dental implants using guided tissue regeneration: a report of two cases[J]. Int J Oral Maxillofac Implants, 1990,5(1):9-14.
pmid: 2391139 |
| [6] |
MacBeth N, Trullenque-Eriksson A, Donos N, et al. Hard and soft tissue changes following alveolar ridge preservation: a syste-matic review[J]. Clin Oral Implants Res, 2017,28(8):982-1004.
doi: 10.1111/clr.12911 pmid: 27458031 |
| [7] | 詹雅琳, 胡文杰, 甄敏, 等. 去蛋白牛骨基质与可吸收胶原膜的磨牙拔牙位点保存效果影像学评价[J]. 北京大学学报(医学版), 2015,47(1):19-26. |
| [8] |
Kim JJ, Schwarz F, Song HY, et al. Ridge preservation of extraction sockets with chronic pathology using Bio-Gide® Collagen with or without collagen membrane: an experimental study in dogs[J]. Clin Oral Implants Res, 2017,28(6):727-733.
doi: 10.1111/clr.12870 pmid: 27194177 |
| [9] |
Wu W, Li B, Liu Y, et al. Effect of multilaminate small intestinal submucosa as a barrier membrane on bone formation in a rabbit mandible defect model[J]. Biomed Res Int, 2018,2018:3270293.
pmid: 30018978 |
| [10] | 吴唯伊, 李博文, 刘玉华, 等. 复层猪小肠黏膜下层可吸收膜的降解性能[J]. 北京大学学报(医学版), 2020,52(3):564-569. |
| [11] |
Eitel F, Klapp F, Jacobson W, et al. Bone regeneration in animals and in man. A contribution to understanding the relative value of animal experiments to human pathophysiology[J]. Arch Orthop Trauma Surg, 1981,99(1):59-64.
pmid: 7316703 |
| [12] |
Lindhe J, Araujo MG, Bufler M, et al. Biphasic alloplastic graft used to preserve the dimension of the edentulous ridge: an experimental study in the dog[J]. Clin Oral Implants Res, 2013,24(10):1158-1163.
pmid: 22804845 |
| [13] |
Naenni N, Sapata V, Bienz SP, et al. Effect of flapless ridge preservation with two different alloplastic materials in sockets with buccal dehiscence defects-volumetric and linear changes[J]. Clin Oral Investig, 2018,22(6):2187-2197.
doi: 10.1007/s00784-017-2309-6 pmid: 29280075 |
| [14] | 詹雅琳, 胡文杰, 徐涛, 等. 罹患重度牙周炎磨牙拔除后应用去蛋白牛骨基质与可吸收胶原膜进行位点保存的组织学研究[J]. 北京大学学报(医学版), 2017,49(1):169-175. |
| [15] |
Benic GI, Thoma DS, Sanz-Martin I, et al. Guided bone regene-ration at zirconia and titanium dental implants: a pilot histological investigation[J]. Clin Oral Implants Res, 2017,28(12):1592-1599.
doi: 10.1111/clr.13030 pmid: 28653343 |
| [16] |
Wang F, Li Q, Wang Z. A comparative study of the effect of Bio-Gide® in combination with concentrated growth factors or bone marrow-derived mesenchymal stem cells in canine sinus grafting[J]. J Oral Pathol Med, 2017,46(7):528-536.
pmid: 27682609 |
| [17] |
Turri A, Elgali I, Vazirisani F, et al. Guided bone regeneration is promoted by the molecular events in the membrane compartment[J]. Biomaterials, 2016,84:167-183.
pmid: 26828682 |
| [18] |
Bouxsein ML, Boyd SK, Christiansen BA, et al. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography[J]. J Bone Miner Res, 2010,25(7):1468-1486.
pmid: 20533309 |
| [19] | Leventis M, Fairbairn P, Mangham C, et al. Bone healing in rabbit calvaria defects using a synthetic bone substitute: A histological and micro-CT comparative study[J]. Materials (Basel), 2018,11(10):1-13. |
| [20] |
Sun Y, Wang CY, Wang ZY, et al. Test in canine extraction site preservations by using mineralized collagen plug with or without membrane[J]. J Biomater Appl, 2016,30(9):1285-1299.
pmid: 26721867 |
| [21] |
Omar O, Dahlin A, Gasser A, et al. Tissue dynamics and rege-nerative outcome in two resorbable non-cross-linked collagen memb-ranes for guided bone regeneration: A preclinical molecular and histological study in vivo[J]. Clin Oral Implants Res, 2018,29(1):7-19.
pmid: 28703398 |
| [1] | Han ZHAO,Yan WEI,Xuehui ZHANG,Xiaoping YANG,Qing CAI,Chengyun NING,Mingming XU,Wenwen LIU,Ying HUANG,Ying HE,Yaru GUO,Shengjie JIANG,Yunyang BAI,Yujia WU,Yusi GUO,Xiaona ZHENG,Wenjing LI,Xuliang DENG. Bionic design, preparation and clinical translation of oral hard tissue restorative materials [J]. Journal of Peking University (Health Sciences), 2024, 56(1): 4-8. |
| [2] | Deng-hui DUAN,Hom-Lay WANG,En-bo WANG. Role of collagen membrane in modified guided bone regeneration surgery using buccal punch flap approach: A retrospective and radiographical cohort study [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 1097-1104. |
| [3] | CHEN Zhen,GU Bao-xin,TANG Yu-fang,YAN Zi-yu,NI Fang-duan,CUI Nian-hui. Constructions of the scale of difficulty in the extraction of impacted mandibular third molars by using Delphi method [J]. Journal of Peking University (Health Sciences), 2022, 54(1): 100-104. |
| [4] | YOU Peng-yue,LIU Yu-hua,WANG Xin-zhi,WANG Si-wen,TANG Lin. Biocompatibility and effect on bone formation of a native acellular porcine pericardium: Results of in vitro and in vivo [J]. Journal of Peking University (Health Sciences), 2021, 53(4): 776-784. |
| [5] | Wei-yi WU,Bo-wen LI,Yu-hua LIU,Xin-zhi WANG. Biodegradation properties of multi-laminated small intestinal submucosa [J]. Journal of Peking University (Health Sciences), 2020, 52(3): 564-569. |
| [6] | Dong SHI,Jie CAO,Shi-ai DAI,Huan-xin MENG. Short-term outcome of regenerative surgery treating peri-implantitis [J]. Journal of Peking University(Health Sciences), 2020, 52(1): 58-63. |
| [7] | CAO Jie, MENG Huan-xin. Evaluation of using cone beam computed tomography as a regular test before and after periodontal regenerative surgery#br# [J]. Journal of Peking University(Health Sciences), 2018, 50(1): 110-116. |
| [8] | XU Xiao, XU Li, JIANG Jiu-hui, WU Jia-qi, LI Xiao-tong, JING Wu-di. Accuracy analysis of alveolar dehiscence and fenestration of maxillary anterior teeth of Angle class Ⅲ by cone-beam CT [J]. Journal of Peking University(Health Sciences), 2018, 50(1): 104-109. |
| [9] | JIA Peng-chen, YANG Gang, HU Wen-jie, ZHAO Yi-jiao, LIU Mu-qing. Preliminary study on the accuracy of infrabony root surface area of single-root teeth by periapical films [J]. Journal of Peking University(Health Sciences), 2018, 50(1): 91-97. |
| [10] | WANG Tian-jiao, LIU Yu, GUAN Ming. Intravenous sedation with midazolam and propofol target controlled infusion on patients’ perioperative anxiety under the mandibular third molar extraction [J]. Journal of Peking University(Health Sciences), 2017, 49(6): 1044-1049. |
| [11] | ZHAO Li-ping, ZHAN Ya-lin, HU Wen-jie, WANG Hao-jie, WEI Yi-ping, ZHEN Min, Xu Tao, LIU Yun-song. Dental implantation and soft tissue augmentation after ridge preservation in a molar site: a case report [J]. Journal of Peking University(Health Sciences), 2016, 48(6): 1090-1094. |
| [12] | LI Dan, GUO Chuan-bin, LIU Yu, WANG En-bo. Applicational evaluation of split tooth extractions of upper molars using piezosurgery [J]. Journal of Peking University(Health Sciences), 2016, 48(4): 709-713. |
| [13] | HUANG Jun-qiang, LIU Shi-yao, JIANG Jiu-hui. Therapeutic evaluation of the correction of the severe bi-maxillary protrusion cases by Tweed-Merrifield technique [J]. Journal of Peking University(Health Sciences), 2016, 48(3): 555-561. |
| [14] | JIANG Xi, LIN Ye, ZHANG Yu, DI Ping, CHEN Bo, HU Xiu-lian, LUO Jia, REN Shu-xin, OUYANG Si-yuan. A novel technique to preserve the alveolar ridge width following tooth extraction in the maxillary frontal area [J]. Journal of Peking University(Health Sciences), 2016, 48(1): 175-179. |
| [15] | ZHANG Jie, LI Xiao-tong. Study of anterior alveolar bone thickness in skeletal class Ⅲ malocclusion patients with orthognathic surgery [J]. Journal of Peking University(Health Sciences), 2016, 48(1): 111-115. |
|
||