Journal of Peking University (Health Sciences) ›› 2025, Vol. 57 ›› Issue (2): 309-316. doi: 10.19723/j.issn.1671-167X.2025.02.014
Previous Articles Next Articles
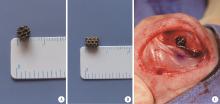
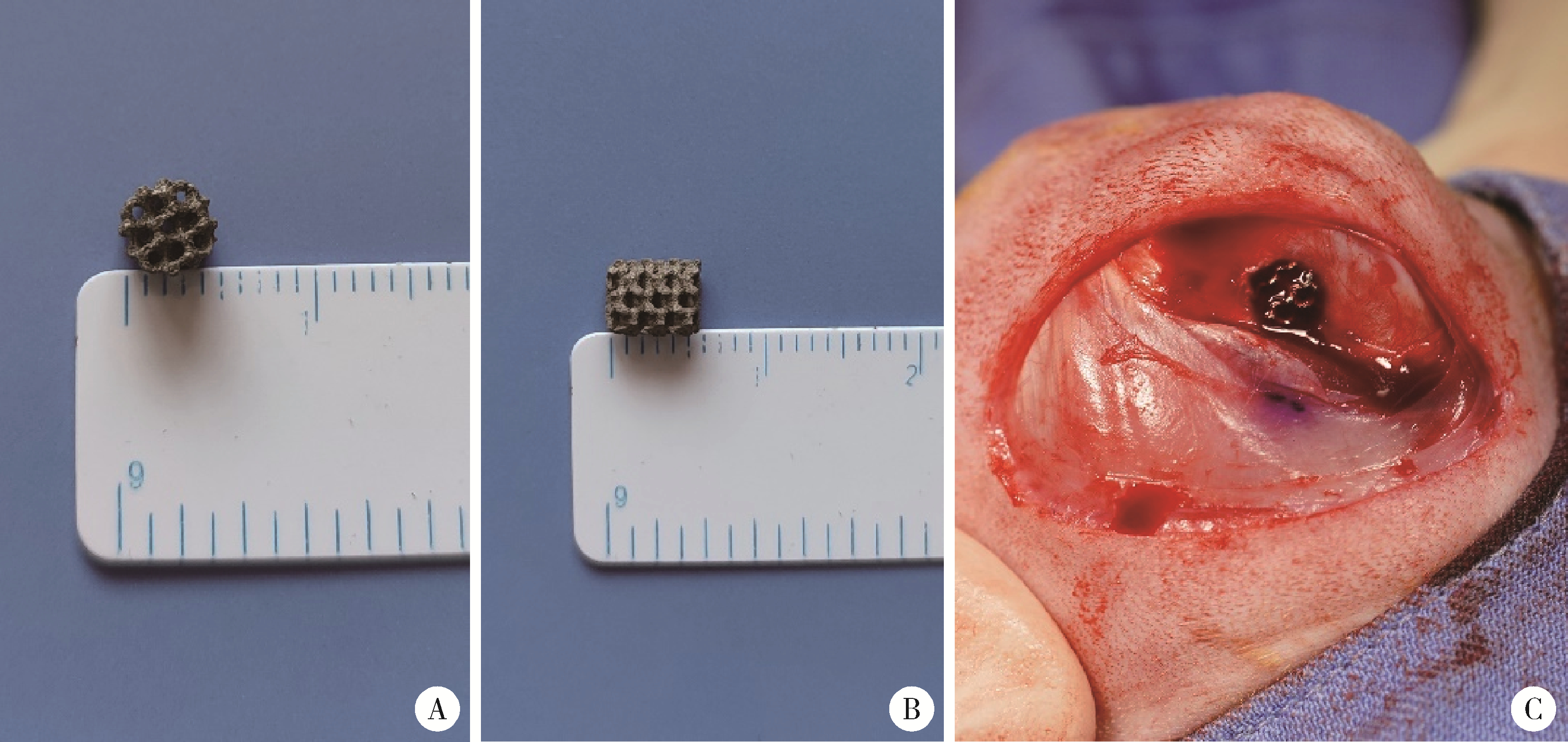
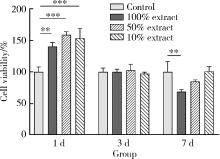
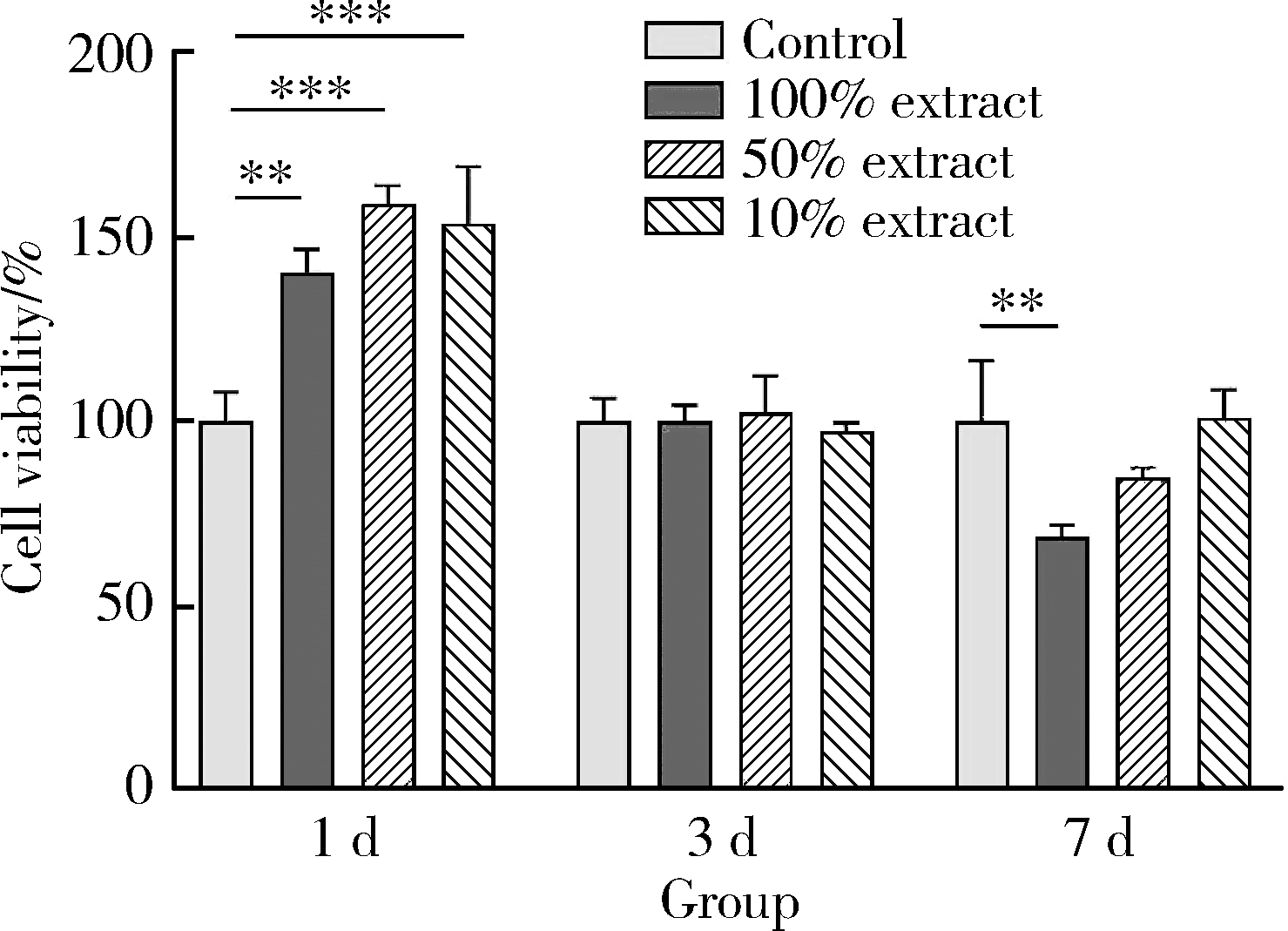
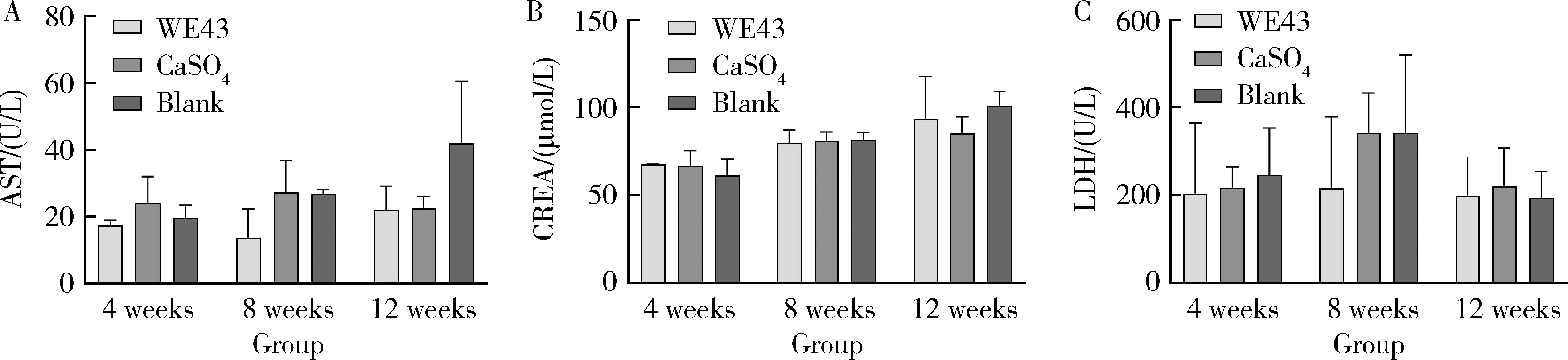
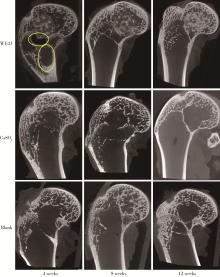
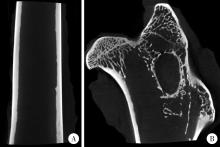
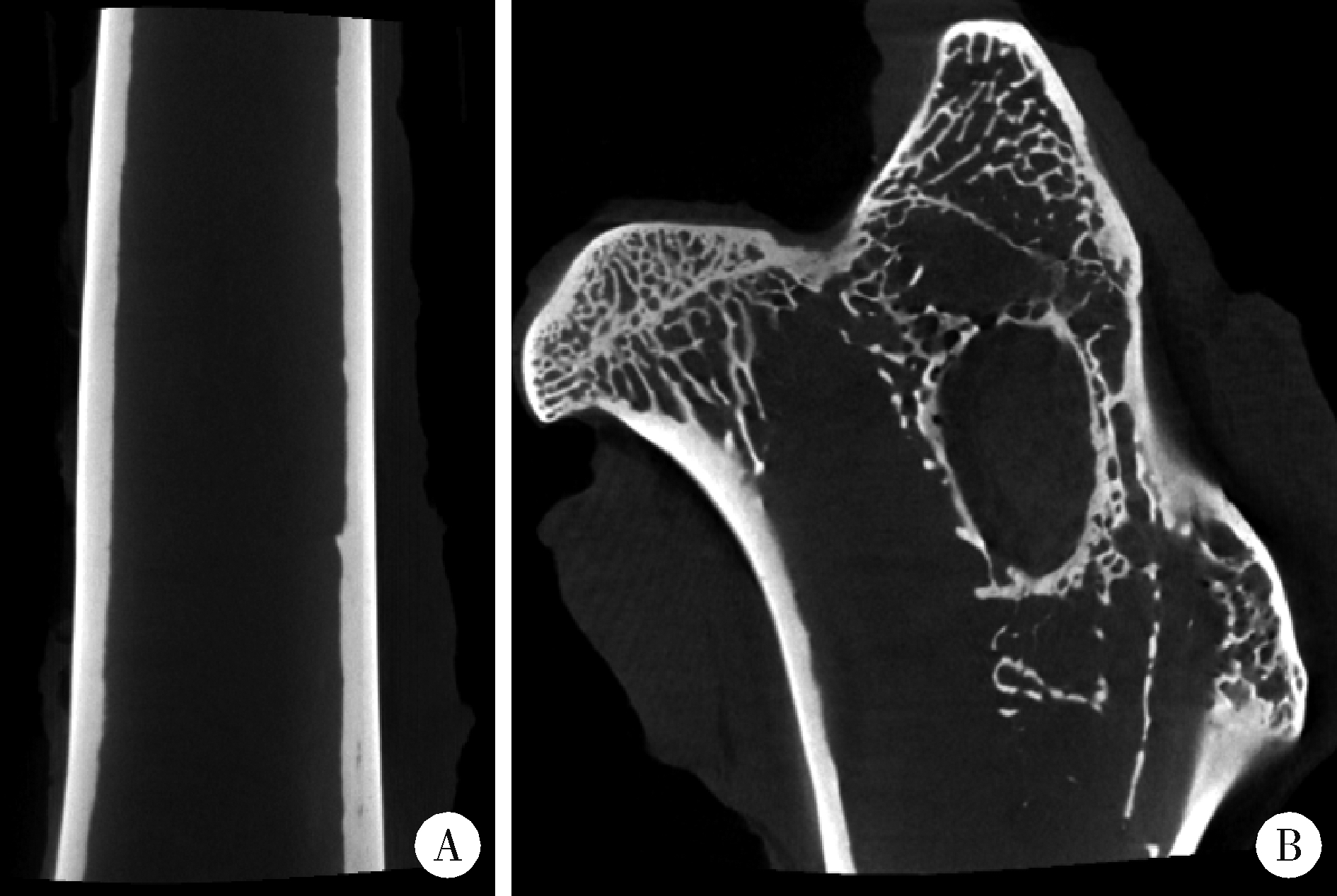
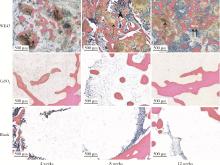
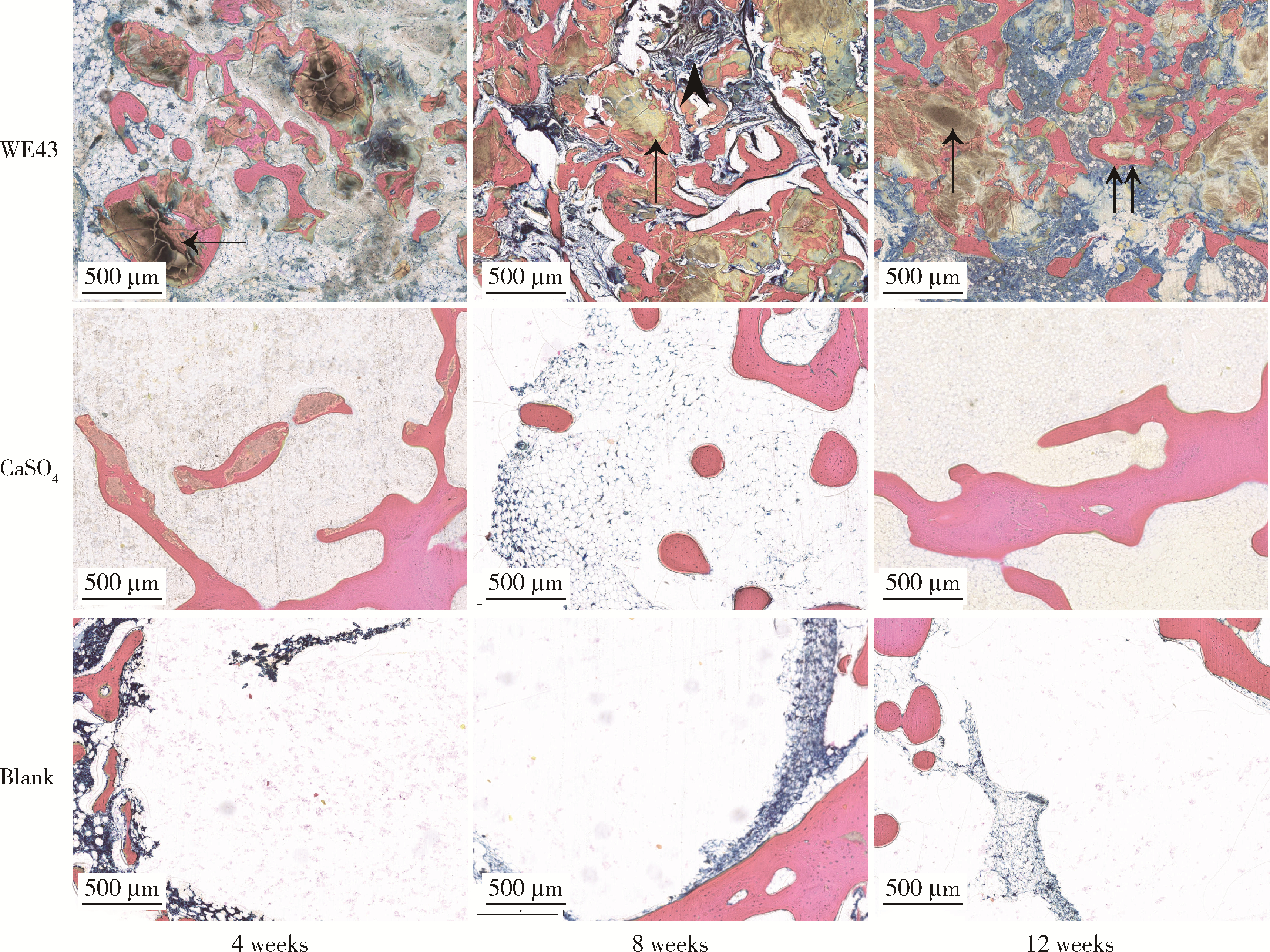
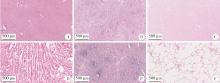
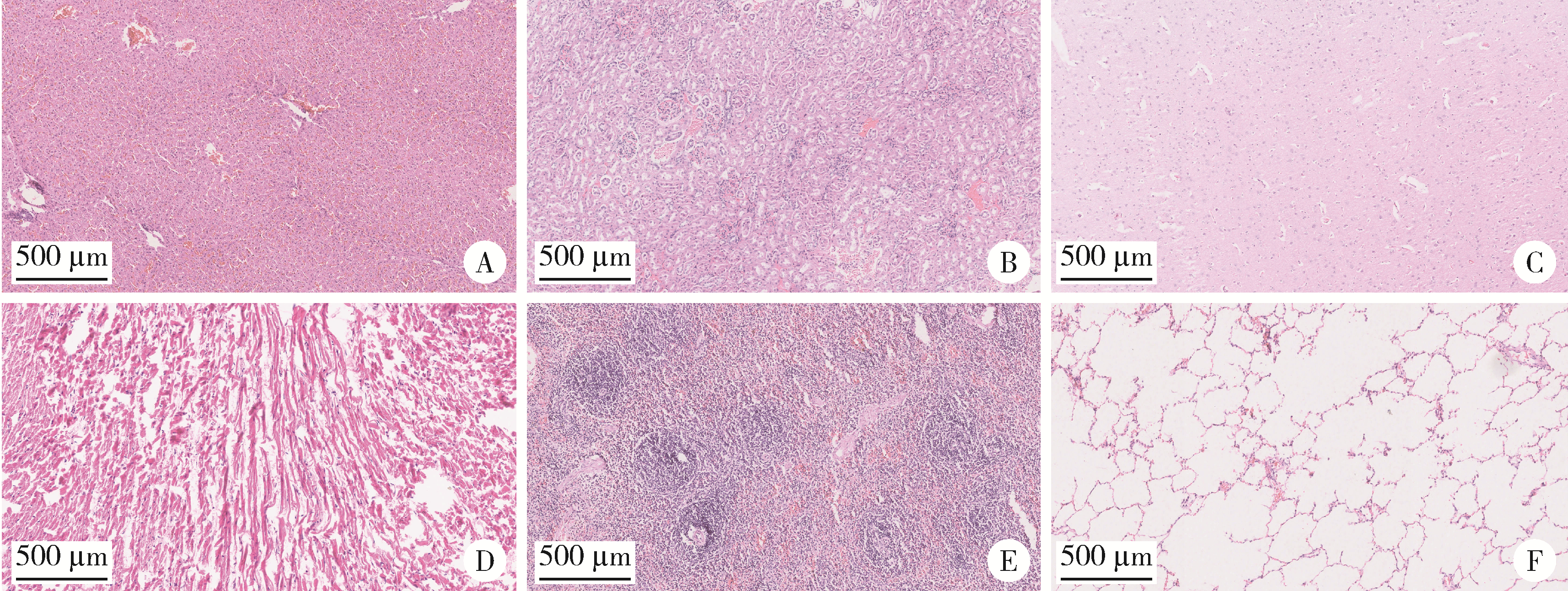
Biocompatibility of 3D printed biodegradable WE43 magnesium alloy scaffolds and treatment of bone defects
- Department of Orthopedics, Peking University Third Hospital; Engineering Research Center of Bone and Joint Precision Medicine, Ministry of Education, Beijing 100191, China
CLC Number:
- R681.8
| 1 | Archunan MW , Petronis S . Bone grafts in trauma and orthopaedics[J]. Cureus, 2021, 13 (9): e17705. |
| 2 |
Grambart ST , Anderson DS , Anderson TD . Bone grafting options[J]. Clin Podiatr Med Surg, 2020, 37 (3): 593- 600.
doi: 10.1016/j.cpm.2020.03.012 |
| 3 |
Lodoso-Torrecilla I , van den Beucken J , Jansen JA . Calcium phosphate cements: Optimization toward biodegradability[J]. Acta Biomater, 2021, 119, 1- 12.
doi: 10.1016/j.actbio.2020.10.013 |
| 4 |
Kani KK , Porrino JA , Chew FS . External fixators: Looking beyond the hardware maze[J]. Skeletal Radiol, 2020, 49 (3): 359- 374.
doi: 10.1007/s00256-019-03306-w |
| 5 |
Deng F , Liu L , Li Z , et al. 3D printed Ti6Al4V bone scaffolds with different pore structure effects on bone ingrowth[J]. J Biol Eng, 2021, 15 (1): 4.
doi: 10.1186/s13036-021-00255-8 |
| 6 |
Sumner DR . Long-term implant fixation and stress-shielding in total hip replacement[J]. J Biomech, 2015, 48 (5): 797- 800.
doi: 10.1016/j.jbiomech.2014.12.021 |
| 7 | Karunakaran R , Ortgies S , Tamayol A , et al. Additive manufacturing of magnesium alloys[J]. Bioact Mater, 2020, 5 (1): 44- 54. |
| 8 | Zhang J , Jiang Y , Shang Z , et al. Biodegradable metals for bone defect repair: A systematic review and meta-analysis based on animal studies[J]. Bioact Mater, 2021, 6 (11): 4027- 4052. |
| 9 | Witte F , Hort N , Vogt C , et al. Degradable biomaterials based on magnesium corrosion[J]. Curr Opin Solid State Mater Sci, 2008, 12 (5/6): 63- 72. |
| 10 | Saris NE , Mervaala E , Karppanen H , et al. Magnesium. An update on physiological, clinical and analytical aspects[J]. Clin Chim Acta, 2000, 294 (1/2): 1- 26. |
| 11 |
Janning C , Willbold E , Vogt C , et al. Magnesium hydroxide temporarily enhancing osteoblast activity and decreasing the osteoclast number in peri-implant bone remodelling[J]. Acta Biomater, 2010, 6 (5): 1861- 1868.
doi: 10.1016/j.actbio.2009.12.037 |
| 12 | Zhang X , Chen Q , Mao X . Magnesium enhances osteogenesis of BMSCs by tuning osteoimmunomodulation[J]. Biomed Res Int, 2019, 2019, 7908205. |
| 13 |
Leem YH , Lee KS , Kim JH , et al. Magnesium ions facilitate integrin alpha 2- and alpha 3-mediated proliferation and enhance alkaline phosphatase expression and activity in hBMSCs[J]. J Tissue Eng Regen Med, 2016, 10 (10): E527- E536.
doi: 10.1002/term.1861 |
| 14 | Chen K , Xie X , Tang H , et al. In vitro and in vivo degradation behavior of Mg-2Sr-Ca and Mg-2Sr-Zn alloys[J]. Bioact Mater, 2020, 5 (2): 275- 285. |
| 15 | Xia D , Liu Y , Wang S , et al. In vitro and in vivo investigation on biodegradable Mg-Li-Ca alloys for bone implant application[J]. Sci China Mater, 2018, 62 (2): 256- 272. |
| 16 | Li Z , Gu X , Lou S , et al. The development of binary Mg-Ca alloys for use as biodegradable materials within bone[J]. Biomaterials, 2008, 29 (10): 1329- 1344. |
| 17 | He LY , Zhang XM , Liu B , et al. Effect of magnesium ion on human osteoblast activity[J]. Braz J Med Biol Res, 2016, 49 (7): e5257. |
| 18 | International Organization for Standardization. Biological evaluation of medical devices. Part 12: Sample preparation and reference materials: ISO 10993-12: 2021[S]. Switzerland: Vernier, 2021: 01. |
| 19 | Morgan EF , Unnikrisnan GU , Hussein AI . Bone mechanical pro-perties in healthy and diseased states[J]. Annu Rev Biomed Eng, 2018, 20, 119- 143. |
| 20 | Cuppone M , Seedhom BB , Berry E , et al. The longitudinal Young's modulus of cortical bone in the midshaft of human femur and its correlation with CT scanning data[J]. Calcif Tissue Int, 2004, 74 (3): 302- 309. |
| 21 | Lu WC , Pringa E , Chou L . Effect of magnesium on the osteogenesis of normal human osteoblasts[J]. Magnes Res, 2017, 30 (2): 42- 52. |
| 22 | Zheng YF , Gu XN , Witte F . Biodegradable metals[J]. Mater Sci Eng R Rep, 2014, 77, 1- 34. |
| 23 | Gu XN , Xie XH , Li N , et al. In vitro and in vivo studies on a Mg-Sr binary alloy system developed as a new kind of biodegradable metal[J]. Acta Biomater, 2012, 8 (6): 2360- 2374. |
| 24 | von der Höh N , von Rechenberg B , Bormann D , et al. Influence of different surface machining treatments of resorbable magnesium alloy implants on degradation-EDX-analysis and histology results[J]. Materwiss Werksttech, 2009, 40 (1/2): 88- 93. |
| 25 | Witte F , Fischer J , Nellesen J , et al. In vitro and in vivo corrosion measurements of magnesium alloys[J]. Biomaterials, 2006, 27 (7): 1013- 1018. |
| 26 | Feyerabend F , Fischer J , Holtz J , et al. Evaluation of short-term effects of rare earth and other elements used in magnesium alloys on primary cells and cell lines[J]. Acta Biomater, 2010, 6 (5): 1834- 1842. |
| 27 | Li F , Gong A , Qiu L , et al. Simultaneous determination of trace rare-earth elements in simulated water samples using ICP-OES with TODGA extraction/back-extraction[J]. PLoS One, 2017, 12 (9): e0185302. |
| 28 | Angrisani N , Reifenrath J , Zimmermann F , et al. Biocompatibility and degradation of LAE442-based magnesium alloys after implantation of up to 3.5 years in a rabbit model[J]. Acta Biomater, 2016, 44, 355- 365. |
| [1] | Xinxin ZHAN,Lulu CAO,Dong XIANG,Hao TANG,Dandan XIA,Hong LIN. Effect of printing orientation on physical and mechanical properties of 3D printing prosthodontic base resin materials [J]. Journal of Peking University (Health Sciences), 2024, 56(2): 345-351. |
| [2] | ABUDUREHEMAN Kaidierya,Rong-geng ZHANG,Hao-nan QIAN,Zhen-yang ZOU,YESITAO Danniya,Tian-yuan FAN. Preparation and in vitro evaluation of FDM 3D printed theophylline tablets with personalized dosage [J]. Journal of Peking University (Health Sciences), 2022, 54(6): 1202-1207. |
| [3] | SUN Yu-chun,GUO Yu-qing,CHEN Hu,DENG Ke-hui,LI Wei-wei. Independent innovation research, development and transformation of precise bionic repair technology for oral prosthesis [J]. Journal of Peking University (Health Sciences), 2022, 54(1): 7-12. |
| [4] | WANG Jing-qi,WANG Xiao. In vivo study of strontium-doped calcium phosphate cement for biological properties [J]. Journal of Peking University (Health Sciences), 2021, 53(2): 378-383. |
| [5] | Fei LI,Jing QIAO,Jin-yu DUAN,Yong ZHANG,Xiu-jing WANG. Effect of concentrated growth factors combined with guided tissue regeneration in treatment of classⅡ furcation involvements of mandibular molars [J]. Journal of Peking University (Health Sciences), 2020, 52(2): 346-352. |
| [6] | Chang CAO,Fei WANG,En-bo WANG,Yu LIU. Application of β-TCP for bone defect restore after the mandibular third molars extraction: A split-mouth clinical trial [J]. Journal of Peking University(Health Sciences), 2020, 52(1): 97-102. |
| [7] | Wei-ting LI,Peng LI,Mu-zi PIAO,Fang ZHANG,Jie DI. Study on bone volume harvested from the implant sites with different methods [J]. Journal of Peking University(Health Sciences), 2020, 52(1): 103-106. |
| [8] | Hao WANG,Yang LIU,Hao-chen LIU,Dong HAN,Hai-lan FENG. Detection and functional analysis of BMP2 gene mutation in patients with tooth agenesis [J]. Journal of Peking University(Health Sciences), 2019, 51(1): 9-15. |
| [9] | ZHANG Da, WANG Lin-chuan, ZHOU Yan-heng, LIU Xiao-mo, LI Jing. Precision of three-dimensional printed brackets#br# [J]. Journal of Peking University(Health Sciences), 2017, 49(4): 704-708. |
| [10] | QIAO Jing, DUAN Jin-yu, CHU Yi, SUN Chang-zhou. Effect of concentrated growth factors on the treatment of degree Ⅱ furcation involvements of mandibular molars [J]. Journal of Peking University(Health Sciences), 2017, 49(1): 36-042. |
|
||