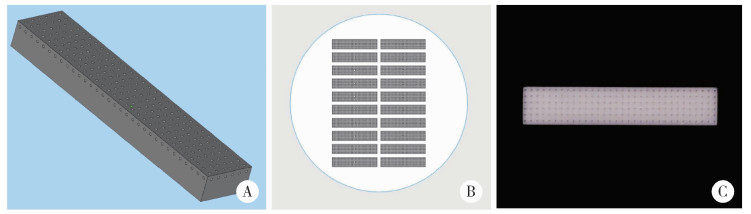
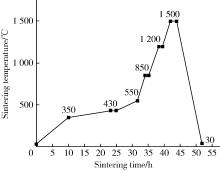
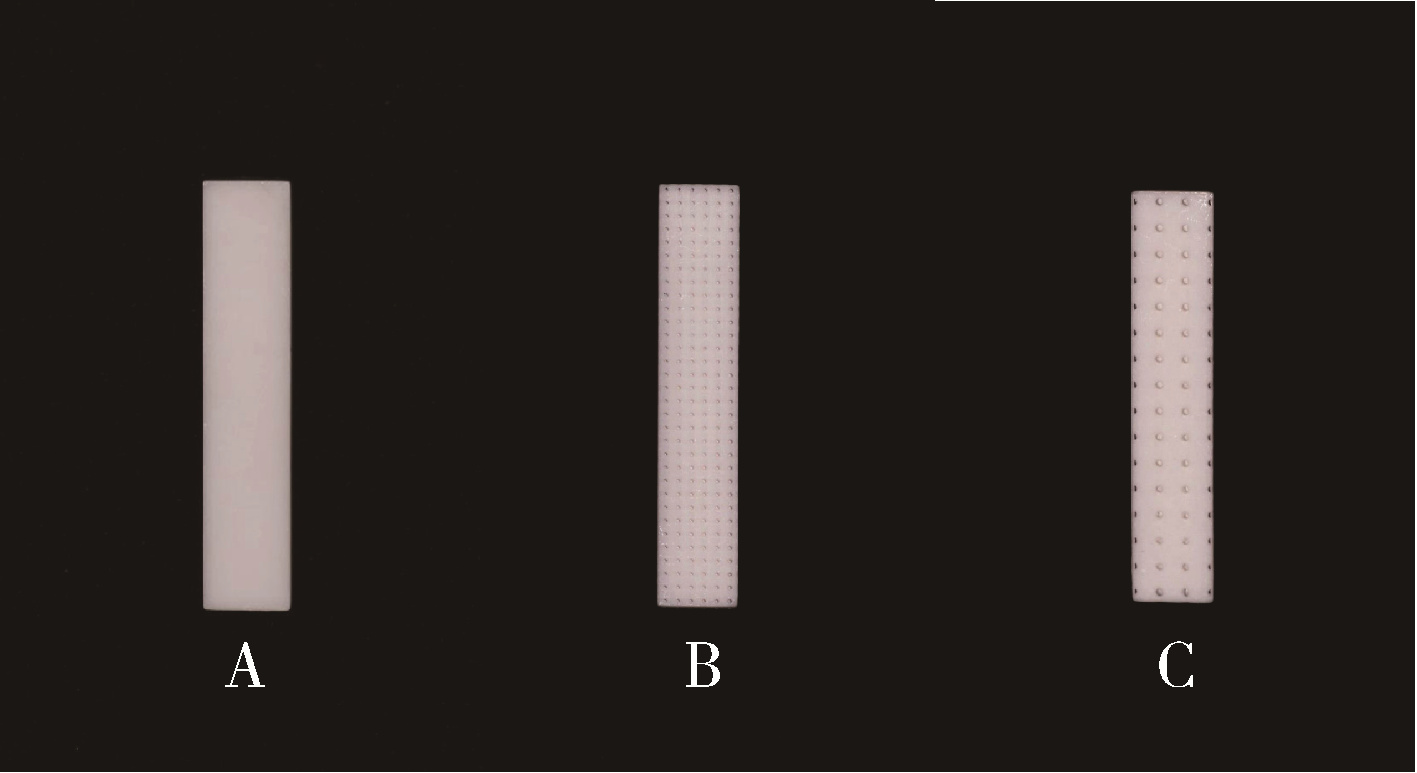
Journal of Peking University (Health Sciences) ›› 2025, Vol. 57 ›› Issue (6): 1165-1173. doi: 10.19723/j.issn.1671-167X.2025.06.022
Previous Articles Next Articles
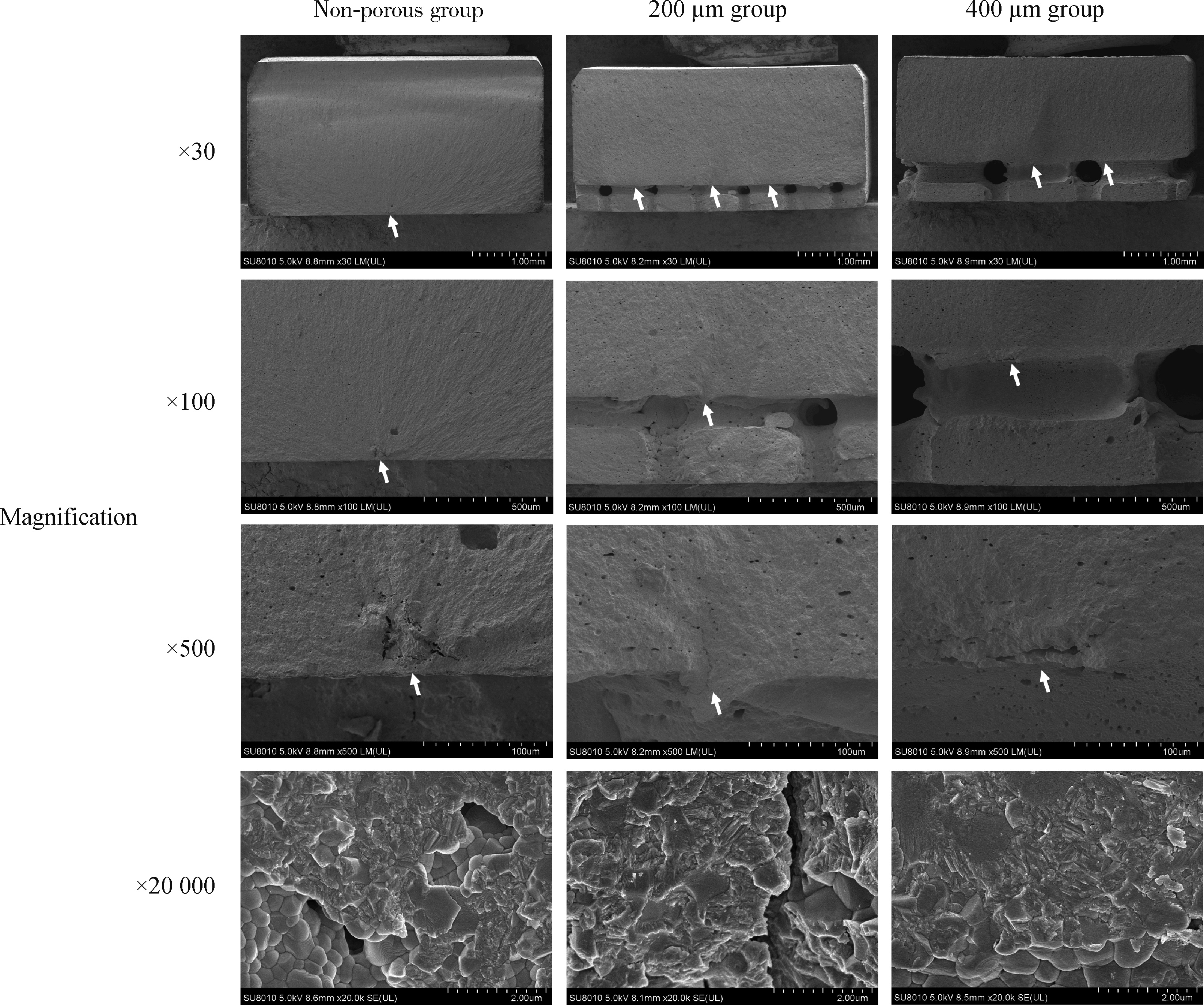
Effect of porous surface structure on fatigue strength of 3D printed zirconia
Jianxiao ZHAO1, Qian DING1, Wenjin LI1, Quanquan MA1, Yixiao LAN2, Lei ZHANG1,*( ), Jianmin HAN2,*(
), Jianmin HAN2,*( )
)
- 1. Department of Prosthodontics, Peking University School and Hospital of Stomatology, Beijing 100081, China
2. Department of Dental Materials, Peking University School and Hospital of Stomatology & National Center for Stomatology & National Clinical Research Center for Oral Diseases & National Engineering Research Center of Oral Biomaterials and Digital Medical Devices & NMPA Key Laboratory for Dental Materials, Beijing 100081, China
CLC Number:
- R783.1
| 1 |
赵祯, 代康, 高勃. 3D打印陶瓷技术在口腔医学领域的研究进展[J]. 中国实用口腔科杂志, 2021, 14 (6): 739- 744.
|
| 2 |
doi: 10.1016/j.jmbbm.2017.08.018 |
| 3 |
doi: 10.1016/j.prosdent.2021.05.010 |
| 4 |
doi: 10.1016/j.msec.2021.111950 |
| 5 |
中国国家标准化管理委员会. 金属平均晶粒度测定方法: GB/T 6394—2017[J]. 北京: 中国标准出版社, 2017, 8- 13.
|
| 6 |
|
| 7 |
doi: 10.1016/j.dental.2005.12.008 |
| 8 |
doi: 10.1016/j.jmbbm.2017.06.016 |
| 9 |
doi: 10.1016/j.jmbbm.2018.05.002 |
| 10 |
doi: 10.1016/j.dental.2017.01.018 |
| 11 |
doi: 10.1016/j.prosdent.2021.11.012 |
| 12 |
doi: 10.1016/j.dental.2013.04.003 |
| 13 |
|
| 14 |
|
| 15 |
doi: 10.1016/0142-9612(96)85758-9 |
| 16 |
doi: 10.1088/1748-6041/10/2/025012 |
| 17 |
doi: 10.1088/1758-5082/6/4/045007 |
| 18 |
doi: 10.3390/nano5020656 |
| 19 |
|
| 20 |
doi: 10.4028/www.scientific.net/MSF.660-661.757 |
| 21 |
doi: 10.1016/j.jmbbm.2014.05.013 |
| 22 |
doi: 10.1016/j.dental.2010.10.025 |
| 23 |
doi: 10.1016/j.pmatsci.2020.100736 |
| 24 |
李文利, 周宏志, 刘卫卫, 等. 光固化3D打印陶瓷浆料及流变性研究进展[J]. 材料工程, 2022, 50 (7): 40- 50.
|
| 25 |
|
| 26 |
doi: 10.3390/ma13061317 |
| 27 |
doi: 10.3390/ma15041602 |
| [1] | Kun QIAN, Yihong LIU. Fitness of onlays fabricated with direct and indirect CAD/CAM technology in vitro [J]. Journal of Peking University (Health Sciences), 2025, 57(3): 604-609. |
| [2] | Shuyuan MIN, Yun TIAN. Biocompatibility of 3D printed biodegradable WE43 magnesium alloy scaffolds and treatment of bone defects [J]. Journal of Peking University (Health Sciences), 2025, 57(2): 309-316. |
| [3] | Xinxin ZHAN,Lulu CAO,Dong XIANG,Hao TANG,Dandan XIA,Hong LIN. Effect of printing orientation on physical and mechanical properties of 3D printing prosthodontic base resin materials [J]. Journal of Peking University (Health Sciences), 2024, 56(2): 345-351. |
| [4] | Qian DING,Wen-jin LI,Feng-bo SUN,Jing-hua GU,Yuan-hua LIN,Lei ZHANG. Effects of surface treatment on the phase and fracture strength of yttria- and magnesia-stabilized zirconia implants [J]. Journal of Peking University (Health Sciences), 2023, 55(4): 721-728. |
| [5] | Wei-wei LI,Hu CHEN,Yong WANG,Yu-chun SUN. Research on friction and wear behaviors of silicon-lithium spray coating on zirconia ceramics [J]. Journal of Peking University (Health Sciences), 2023, 55(1): 94-100. |
| [6] | ABUDUREHEMAN Kaidierya,Rong-geng ZHANG,Hao-nan QIAN,Zhen-yang ZOU,YESITAO Danniya,Tian-yuan FAN. Preparation and in vitro evaluation of FDM 3D printed theophylline tablets with personalized dosage [J]. Journal of Peking University (Health Sciences), 2022, 54(6): 1202-1207. |
| [7] | Yi DENG,Yi ZHANG,Bo-wen LI,Mei WANG,Lin TANG,Yu-hua LIU. Effects of different crosslinking treatments on the properties of decellularized small intestinal submucosa porous scaffolds [J]. Journal of Peking University (Health Sciences), 2022, 54(3): 557-564. |
| [8] | SUN Yu-chun,GUO Yu-qing,CHEN Hu,DENG Ke-hui,LI Wei-wei. Independent innovation research, development and transformation of precise bionic repair technology for oral prosthesis [J]. Journal of Peking University (Health Sciences), 2022, 54(1): 7-12. |
| [9] | WANG Zheng,DING Qian,GAO Yuan,MA Quan-quan,ZHANG Lei,GE Xi-yuan,SUN Yu-chun,XIE Qiu-fei. Effect of porous zirconia ceramics on proliferation and differentiation of osteoblasts [J]. Journal of Peking University (Health Sciences), 2022, 54(1): 31-39. |
| [10] | LI Wen-jin,DING Qian,YUAN Fu-song,Sun Feng-bo,ZHENG Jian-qiao,BAO Rui,Zhang Lei. Effects of femtosecond laser treatment on surface characteristics and flexural strength of zirconia [J]. Journal of Peking University (Health Sciences), 2021, 53(4): 770-775. |
| [11] | YANG Xin,LI Rong,YE Hong-qiang,CHEN Hu,WANG Yong,ZHOU Yong-sheng,SUN Yu-chun. Evaluation of fracture strength of two kinds of zirconia all-ceramic crowns with different edge compensation angles [J]. Journal of Peking University (Health Sciences), 2021, 53(2): 402-405. |
| [12] | Miao ZHENG,Ling-lu ZHAN,Zhi-qiang LIU,He-ping LI,Jian-guo TAN. Effect of different plasma treated zirconia on the adhensive behaviour of human gingival fibroblasts [J]. Journal of Peking University(Health Sciences), 2019, 51(2): 315-320. |
| [13] | ZHOU Tuan-feng, WANG Xin-zhi . Clinical observation of the restoration of computer aided designed and manufactured one-piece zirconia posts and cores: a 5-year prospective follow-up study [J]. Journal of Peking University(Health Sciences), 2018, 50(4): 680-684. |
| [14] | ZHU Lin, WANG Yu-dong, DONG Yan-mei,CHEN Xiao-feng. Mesoporous nano-bioactive glass microspheres as a drug delivery system of mino-cycline [J]. Journal of Peking University(Health Sciences), 2018, 50(2): 249-255. |
| [15] | CUI Xin-yue, TONG Dai, WANG Xin-zhi, SHEN Zhi-jian. Comparison of the translucency and color masking effect of the zirconia ceramics made by milling and gel deposition [J]. Journal of Peking University(Health Sciences), 2018, 50(1): 85-90. |
|
||