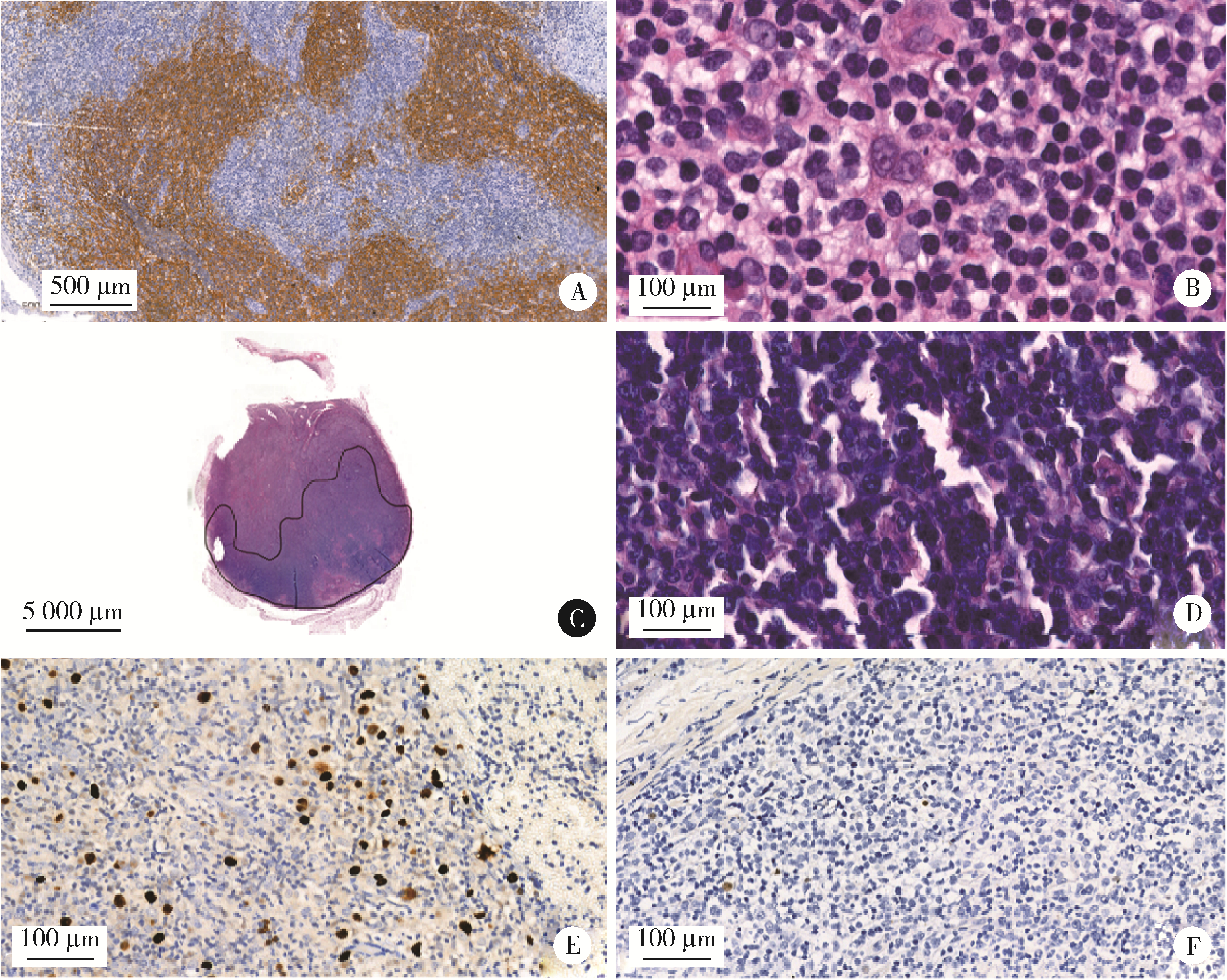
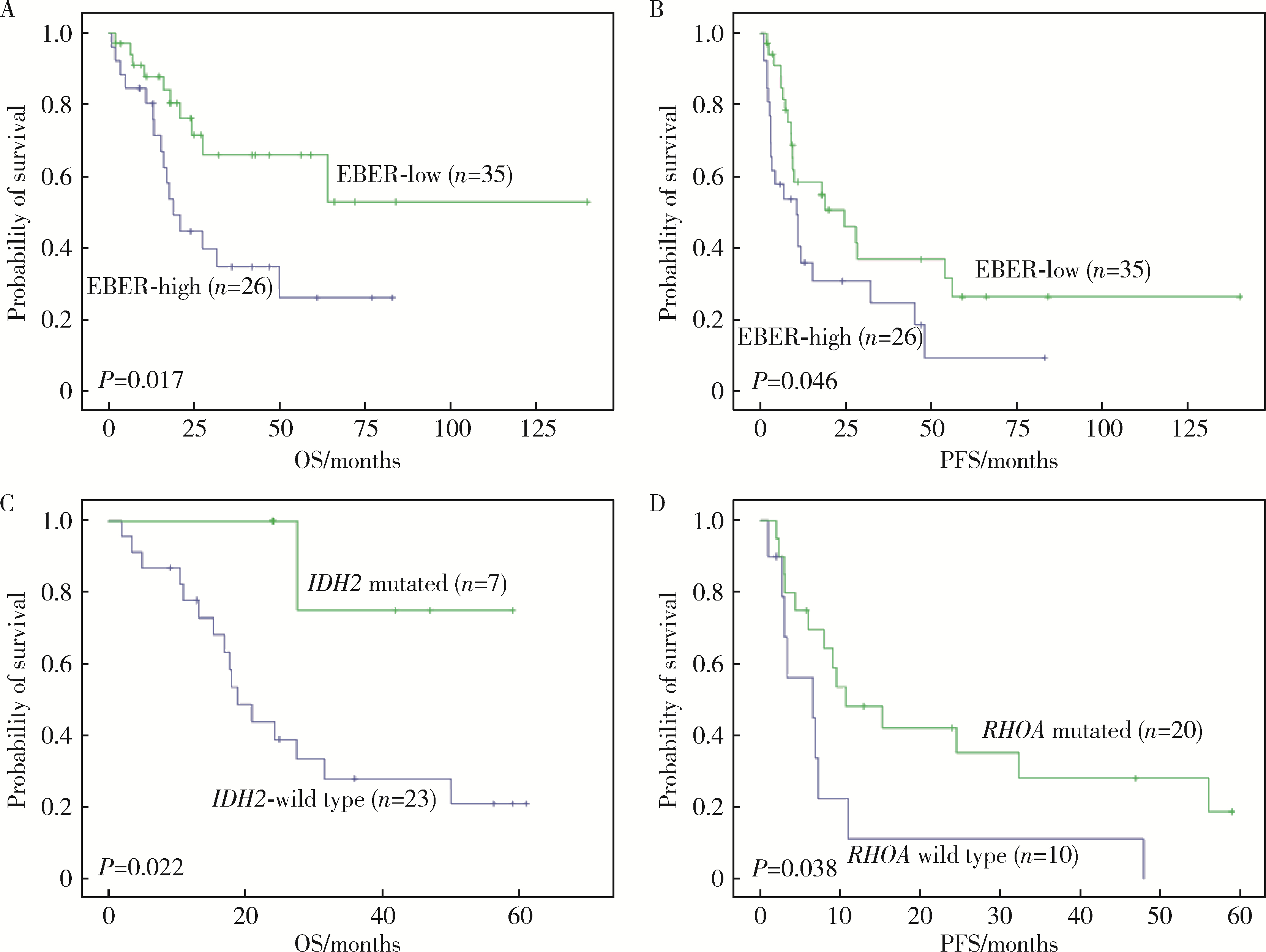
Journal of Peking University (Health Sciences) ›› 2023, Vol. 55 ›› Issue (3): 521-529. doi: 10.19723/j.issn.1671-167X.2023.03.019
Previous Articles Next Articles
Analysis of clinicopathological and molecular abnormalities of angioimmunoblastic T-cell lymphoma
Yun-fei SHI1,Hao-jie WANG2,Wei-ping LIU3,Lan MI3,Meng-ping LONG1,Yan-fei LIU3,Yu-mei LAI1,Li-xin ZHOU1,Xin-ting DIAO1,Xiang-hong LI1,*( )
)
- 1. Department of Pathology, Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education), Peking University Cancer Hospital & Institute, Beijing 100142, China
2. Central Laboratory, Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education), Peking University Cancer Hospital & Institute, Beijing 100142, China
3. Department of Lymphoma, Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education), Peking University Cancer Hospital & Institute, Beijing 100142, China
CLC Number:
- R732.2
| 1 |
Swerdlow SH , Campo E , Pileri SA , et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms[J]. Blood, 2016, 127 (20): 2375- 2390.
doi: 10.1182/blood-2016-01-643569 |
| 2 |
Laurent C , Baron M , Amara N , et al. Impact of expert pathologic review of lymphoma diagnosis: Study of patients from the French lymphopath network[J]. J Clin Oncol, 2017, 35 (18): 2008- 2017.
doi: 10.1200/JCO.2016.71.2083 |
| 3 | van Krieken JHJM , Langerak AW , Macintyre EA , et al. Improved reliability of lymphoma diagnostics via PCR-based clonality testing: Report of the BIOMED-2 concerted action BHM4-CT98-3936[J]. Leukemia, 2006, 21 (2): 201- 206. |
| 4 |
Langerak AW , Groenen PJTA , Brüggemann M , et al. Euro-Clonality/BIOMED-2 guidelines for interpretation and reporting of Ig/TCR clonality testing in suspected lymphoproliferations[J]. Leukemia, 2012, 26 (10): 2159- 2171.
doi: 10.1038/leu.2012.246 |
| 5 |
Cortes JR , Palomero T . The curious origins of angioimmunoblastic T-cell lymphoma[J]. Curr Opin Hematol, 2016, 23 (4): 434- 443.
doi: 10.1097/MOH.0000000000000261 |
| 6 | 郭艳敏, 刘雪霏, 焦莉娟, 等. 血管免疫母细胞性T细胞淋巴瘤组织学分级与预后分析[J]. 中华病理学杂志, 2019, 48 (10): 784- 790. |
| 7 |
Zhang C , Mi L , Wu M , et al. Angioimmunoblastic T-cell lymphoma: Treatment strategies and prognostic factors from a retrospective multicenter study in China[J]. Leuk Lymphoma, 2022, 63 (5): 1152- 1159.
doi: 10.1080/10428194.2021.2015586 |
| 8 |
Hsu YT , Wang YC , Chen RY , et al. Angioimmunoblastic T-cell lymphoma in Taiwan reveals worse progression-free survival for RHOA G17V mutated subtype[J]. Leuk Lymphoma, 2020, 61 (5): 1108- 1118.
doi: 10.1080/10428194.2019.1702179 |
| 9 | 李婷婷, 罗璐婷, 陈溢, 等. 84例血管免疫母细胞性T细胞淋巴瘤的临床特征及预后: 单中心分析[J]. 中华血液学杂志, 2020, 41 (11): 915- 920. |
| 10 |
Steinhilber J , Mederake M , Bonzheim I , et al. The pathological features of angioimmunoblastic T-cell lymphomas with IDH2(R172) mutations[J]. Mod Pathol, 2019, 32 (8): 1123- 1134.
doi: 10.1038/s41379-019-0254-4 |
| 11 |
Hsi ED , Said J , Macon WR , et al. Diagnostic accuracy of a defined immunophenotypic and molecular genetic approach for peripheral T/NK-cell lymphomas. A North American PTCL study group project[J]. Am J Surg Pathol, 2014, 38 (6): 768- 775.
doi: 10.1097/PAS.0000000000000188 |
| 12 |
Heavican TB , Bouska A , Yu J , et al. Genetic drivers of oncogenic pathways in molecular subgroups of peripheral T-cell lymphoma[J]. Blood, 2019, 133 (15): 1664- 1676.
doi: 10.1182/blood-2018-09-872549 |
| 13 |
Schwartz FH , Cai Q , Fellmann E , et al. TET2 mutations in B cells of patients affected by angioimmunoblastic T-cell lymphoma[J]. J Pathol, 2017, 242 (2): 129- 133.
doi: 10.1002/path.4898 |
| 14 |
Palomero T , Couronne L , Khiabanian H , et al. Recurrent mutations in epigenetic regulators, RHOA and FYN kinase in peripheral T cell lymphomas[J]. Nature Genetics, 2014, 46 (2): 166.
doi: 10.1038/ng.2873 |
| 15 |
Yoo HY , Sung MK , Lee SH , et al. A recurrent inactivating mutation in RHOA GTPase in angioimmunoblastic T cell lymphoma[J]. Nat Genet, 2014, 46 (4): 371- 375.
doi: 10.1038/ng.2916 |
| 16 |
Fukumoto K , Nguyen TB , Chiba S , et al. Review of the biologic and clinical significance of genetic mutations in angioimmunoblastic T-cell lymphoma[J]. Cancer Sci, 2018, 109 (3): 490- 496.
doi: 10.1111/cas.13393 |
| 17 |
Chiba S , Sakata-Yanagimoto M . Advances in understanding of angioimmunoblastic T-cell lymphoma[J]. Leukemia, 2020, 34 (10): 2592- 2606.
doi: 10.1038/s41375-020-0990-y |
| 18 | 中国临床肿瘤学会肿瘤标志物专家委员会, 中国肿瘤驱动基因分析联盟. 二代测序技术在肿瘤精准医学诊断中的应用专家共识[J]. 中华医学杂志, 2018, 98 (26): 2057- 2065. |
| 19 | 中国抗癌协会血液肿瘤专业委员会, 中华医学会血液学分会, 中华医学会病理学分会. 二代测序技术在血液肿瘤中的应用中国专家共识(2018年版)[J]. 中华血液学杂志, 2018, 39 (11): 881- 886. |
| 20 |
Ondrejka SL , Grzywacz B , Bodo J , et al. Angioimmunoblastic T-cell lymphomas with the RHOA p.Gly17Val mutation have classic clinical and pathologic features[J]. Am J Surg Pathol, 2016, 40 (3): 335- 341.
doi: 10.1097/PAS.0000000000000555 |
| 21 | 张晨, 王小沛, 郑文, 等. 血管免疫母细胞性T细胞淋巴瘤42例临床分析[J]. 中华医学杂志, 2013, 93 (46): 3671- 3674. |
| 22 | Eladl AE , Shimada K , Suzuki Y , et al. EBV status has prognostic implication among young patients with angioimmunoblastic T-cell lymphoma[J]. Cancer Med, 2020, 9 (2): 678- 688. |
| 23 | 王芳, 张瑰红, 丁凯阳, 等. EBER、PTEN和VEGF在血管免疫母T细胞淋巴瘤中的表达及其临床病理学意义[J]. 中国实验血液学杂志, 2015, 23 (3): 663- 668. |
| 24 | Liang JH , Lu L , Zhu HY , et al. The prognostic role of circulating Epstein-Barr Virus DNA copy number in angioimmunoblastic T-cell lymphoma treated with dose-adjusted EPOCH[J]. Cancer Res Treat, 2019, 51 (1): 150- 157. |
| 25 | Kim TY , Min GJ , Jeon YW , et al. Impact of Epstein-Barr virus on peripheral T-cell lymphoma not otherwise specified and angioimmunoblastic T-cell lymphoma[J]. Front Oncol, 2021, 11, 797028. |
| [1] | Dongwu LIU, Jie CHEN, Mingli GAO, Jing YU. Rheumatoid arthritis with Castleman-like histopathology in lymph nodes: A case report [J]. Journal of Peking University (Health Sciences), 2024, 56(5): 928-931. |
| [2] | Junyong OU,Kunming NI,Lulin MA,Guoliang WANG,Ye YAN,Bin YANG,Gengwu LI,Haodong SONG,Min LU,Jianfei YE,Shudong ZHANG. Prognostic factors of patients with muscle invasive bladder cancer with intermediate-to-high risk prostate cancer [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 582-588. |
| [3] | Shuai LIU,Lei LIU,Zhuo LIU,Fan ZHANG,Lulin MA,Xiaojun TIAN,Xiaofei HOU,Guoliang WANG,Lei ZHAO,Shudong ZHANG. Clinical treatment and prognosis of adrenocortical carcinoma with venous tumor thrombus [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 624-630. |
| [4] | Le YU,Shaohui DENG,Fan ZHANG,Ye YAN,Jianfei YE,Shudong ZHANG. Clinicopathological characteristics and prognosis of multilocular cystic renal neoplasm of low malignant potential [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 661-666. |
| [5] | Zezhen ZHOU,Shaohui DENG,Ye YAN,Fan ZHANG,Yichang HAO,Liyuan GE,Hongxian ZHANG,Guoliang WANG,Shudong ZHANG. Predicting the 3-year tumor-specific survival in patients with T3a non-metastatic renal cell carcinoma [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 673-679. |
| [6] | Yangyi FANG,Qiang LI,Zhigao HUANG,Min LU,Kai HONG,Shudong ZHANG. Well-differentiated papillary mesothelial tumour of the tunica vaginalis: A case report [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 741-744. |
| [7] | Huina HUANG,Jing ZHAO,Xiangge ZHAO,Ziran BAI,Xia LI,Guan WANG. Regulatory effect of lactate on peripheral blood CD4+ T cell subsets in patients with rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2024, 56(3): 519-525. |
| [8] | Yuanyuan ZENG,Yun XIE,Daonan CHEN,Ruilan WANG. Related factors of euthyroid sick syndrome in patients with sepsis [J]. Journal of Peking University (Health Sciences), 2024, 56(3): 526-532. |
| [9] | Jian-bin LI,Meng-na LYU,Qiang CHI,Yi-lin PENG,Peng-cheng LIU,Rui WU. Early prediction of severe COVID-19 in patients with Sjögren’s syndrome [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 1007-1012. |
| [10] | Huan-rui LIU,Xiang PENG,Sen-lin LI,Xin GOU. Risk modeling based on HER-2 related genes for bladder cancer survival prognosis assessment [J]. Journal of Peking University (Health Sciences), 2023, 55(5): 793-801. |
| [11] | Zi-xuan XUE,Shi-ying TANG,Min QIU,Cheng LIU,Xiao-jun TIAN,Min LU,Jing-han DONG,Lu-lin MA,Shu-dong ZHANG. Clinicopathologic features and prognosis of young renal tumors with tumor thrombus [J]. Journal of Peking University (Health Sciences), 2023, 55(5): 802-811. |
| [12] | Han LU,Jian-yun ZHANG,Rong YANG,Le XU,Qing-xiang LI,Yu-xing GUO,Chuan-bin GUO. Clinical factors affecting the prognosis of lower gingival squamous cell carcinoma [J]. Journal of Peking University (Health Sciences), 2023, 55(4): 702-707. |
| [13] | Xiao-juan ZHU,Hong ZHANG,Shuang ZHANG,Dong LI,Xin LI,Ling XU,Ting LI. Clinicopathological features and prognosis of breast cancer with human epidermal growth factor receptor 2 low expression [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 243-253. |
| [14] | Yu-mei LAI,Zhong-wu LI,Huan LI,Yan WU,Yun-fei SHI,Li-xin ZHOU,Yu-tong LOU,Chuan-liang CUI. Clinicopathological features and prognosis of anorectal melanoma: A report of 68 cases [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 262-269. |
| [15] | Qi SHEN,Yi-xiao LIU,Qun HE. Mucinous tubular and spindle cell carcinoma of kidney: Clinicopathology and prognosis [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 276-282. |
|
||