北京大学学报(医学版) ›› 2022, Vol. 54 ›› Issue (5): 981-990. doi: 10.19723/j.issn.1671-167X.2022.05.027
副肿瘤性天疱疮合并实体肿瘤的危重症患者术后远期结局的影响因素
- 1. 北京大学第一医院重症医学科, 北京 100034
2. 北京大学第一医院医学统计室, 北京 100034
3. 北京大学第一医院麻醉科, 北京 100034
Factors associated with long-term survival in critically ill patients following surgery for solid tumors complicated with paraneoplastic pemphigus
Jia-xin PAN1,Sai-nan ZHU2,Shuang-ling LI1,*( ),Dong-xin WANG1,3
),Dong-xin WANG1,3
- 1. Department of Critical Care Medicine, Peking University First Hospital, Beijing 100034, China
2. Department of Biostatistics, Peking University First Hospital, Beijing 100034, China
3. Department of Anesthesiology, Peking University First Hospital, Beijing 100034, China
摘要:
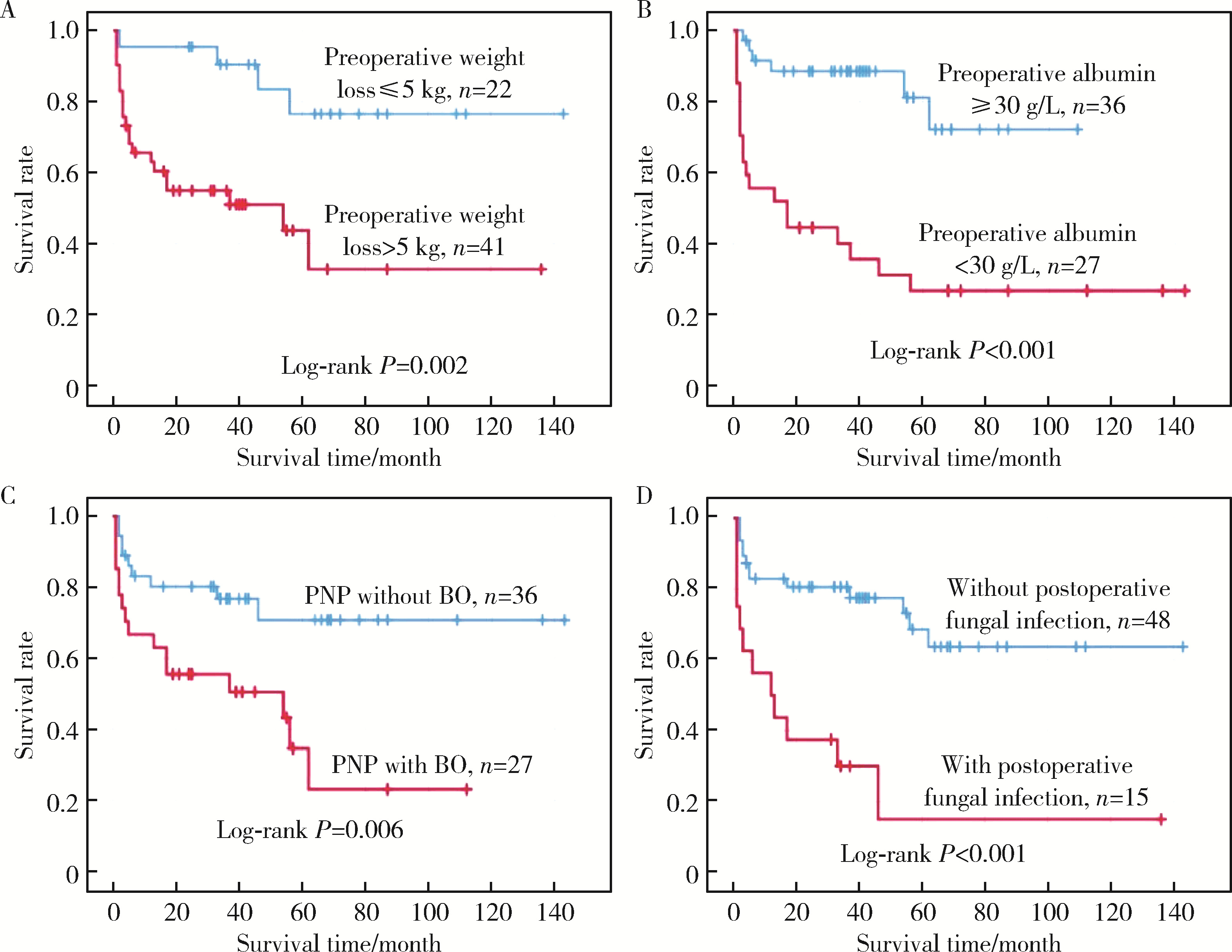
目的: 副肿瘤性天疱疮合并实体肿瘤的重症患者通常需要在手术后收住重症医学科(intensive care unit,ICU)治疗,这类患者的长期死亡率较高。本研究对这类患者的临床特点及远期预后的影响因素进行分析。方法: 回顾性分析2005年1月至2020年12月间63例副肿瘤性天疱疮合并实体肿瘤、手术后收住ICU重症患者的临床和实验室资料,并对患者的生存状况进行随访。结果: 63例患者中,原发肿瘤为Castleman病的占79.4%,其他病理类型为20.6%;皮损程度在重度-广泛的占69.8%,其他皮损程度占30.2%;合并闭塞性细支气管炎的占44.4%,不合并的占55.6%。23.8%的患者并发术后真菌感染,无真菌感染占76.2%。术后中位随访时间为95个月,25例患者在研究期间死亡,术后1年、3年、5年生存率分别为74.6%(95%CI 63.8%~85.4%)、67.4%(95%CI 55.6%~79.2%)和55.1%(95%CI 47.9%~62.3%)。通过对分类因素采用Log-rank法进行单因素分析结果表明:年龄>40岁(P=0.042)、发病后体质量下降>5 kg(P=0.002)、术前白蛋白 < 30 g/L(P < 0.001)、并发闭塞性细支气管炎(P=0.002)、围术期存在真菌感染(P<0.001)的患者死亡率增加;Cox单因素分析显示术前体质量下降>5 kg(P=0.005)、术前白蛋白 < 30 g/L(P < 0.001)、术前合并闭塞性细支气管炎(P=0.009)、术前肺部细菌感染(P=0.007)、手术时间长(P=0.048)、术后入ICU时氧合指数(P=0.012)和白蛋白(P=0.010)、血红蛋白浓度低(P=0.035)、入ICU后急性生理学及慢性健康状态评分(acute physiology and chronic health evaluation, APACHE Ⅱ, P=0.001)、序贯器官衰竭评分(sequential organ failure assessment, SOFA, P=0.010),以及术后真菌感染都是影响远期存活的危险因素(P < 0.001)。Cox回归模型进行多因素分析结果表明,术前体质量下降>5 kg(HR 4.44; 95%CI 1.47~13.38; P=0.008)、术前白蛋白 < 30 g/L(HR 4.38; 95%CI 1.72~11.12; P=0.002)、术前合并闭塞性细支气管炎(HR 2.69; 95%CI 1.12~6.50; P=0.027)及术后并发真菌感染(HR 4.85; 95%CI 2.01-11.72; P<0.001)是术后死亡的独立危险因素。结论: 因副肿瘤性天疱疮合并实体肿瘤而接受手术治疗的重症患者术后的5年存活率约为55.1%,术前体质量下降>5 kg、白蛋白 < 30 g/L、合并闭塞性细支气管炎和术后并发真菌感染是术后近远期死亡风险增加的影响因素。
中图分类号:
- R593.2
| 1 |
Anhalt GJ , Kim SC , Stanley JR , et al. Paraneoplastic pemphigus. An autoimmune mucocutaneous disease associated with neoplasia[J]. N Engl J Med, 1990, 323 (25): 1729- 1735.
doi: 10.1056/NEJM199012203232503 |
| 2 | Nguyen VT , Ndoye A , Bassler KD , et al. Classification, clinical manifestations, and immunopathological mechanisms of the epithelial variant of paraneoplastic autoimmune multiorgan syndrome: a reappraisal of paraneoplastic pemphigus[J]. Arch Dermatol, 2001, 137 (2): 193- 206. |
| 3 |
Paolino G , Didona D , Magliulo G , et al. Paraneoplastic pemphigus: insight into the autoimmune pathogenesis, clinical features and therapy[J]. Int J Mol Sci, 2017, 18 (12): 2532.
doi: 10.3390/ijms18122532 |
| 4 |
Solimani F , Maglie R , Pollmann R , et al. Thymoma-associated paraneoplastic autoimmune multiorgan syndrome: from pemphigus to lichenoid dermatitis[J]. Front Immunol, 2019, 10, 1413.
doi: 10.3389/fimmu.2019.01413 |
| 5 |
Jelti L , Cordel N , Gillibert A , et al. Incidence and mortality of pemphigus in France[J]. J Invest Dermatol, 2019, 139 (2): 469- 473.
doi: 10.1016/j.jid.2018.07.042 |
| 6 |
Cao L , Wang F , Du XY , et al. Chronic lymphocytic leukemia-associated paraneoplastic pemphigus: potential cause and therapeutic strategies[J]. Sci Rep, 2020, 10 (1): 16357.
doi: 10.1038/s41598-020-73131-y |
| 7 |
Tirado-Sanchez A , Bonifaz A . Paraneoplastic pemphigus. A life-threatening autoimmune blistering disease[J]. Actas Dermosifi-liogr, 2017, 108 (10): 902- 910.
doi: 10.1016/j.ad.2017.04.024 |
| 8 |
Kishi T , Nakata J , Yamada T , et al. Fatal progression of bron-chiolitis obliterans in spite of complete remission of follicular lymphoma and paraneoplastic pemphigus[J]. Ann Hematol, 2022, 101 (2): 453- 455.
doi: 10.1007/s00277-021-04499-8 |
| 9 |
Tsuchisaka A , Numata S , Teye K , et al. Epiplakin is a paraneoplastic pemphigus autoantigen and related to bronchiolitis obliterans in Japanese patients[J]. J Invest Dermatol, 2016, 136 (2): 399- 408.
doi: 10.1038/JID.2015.408 |
| 10 |
Aguilar PR , Michelson AP , Isakow W . Obliterative bronchiolitis[J]. Transplantation, 2016, 100 (2): 272- 283.
doi: 10.1097/TP.0000000000000892 |
| 11 |
Krain RL , Kushner CJ , Tarazi M , et al. Assessing the correlation between disease severity indices and quality of life measurement tools in pemphigus[J]. Front Immunol, 2019, 10, 2571.
doi: 10.3389/fimmu.2019.02571 |
| 12 |
Czernik A , Camilleri M , Pittelkow MR , et al. Paraneoplastic autoimmune multiorgan syndrome: 20 years after[J]. Int J Dermatol, 2011, 50 (8): 905- 914.
doi: 10.1111/j.1365-4632.2011.04868.x |
| 13 |
Yong AA , Tey HL . Paraneoplastic pemphigus[J]. Australas J Dermatol, 2013, 54 (4): 241- 250.
doi: 10.1111/j.1440-0960.2012.00921.x |
| 14 | Wang J , Zhu X , Li R , et al. Paraneoplastic pemphigus associated with Castleman tumor: a commonly reported subtype of paraneoplastic pemphigus in China[J]. Arch Dermatol, 2005, 141 (10): 1285- 1293. |
| 15 |
Leger S , Picard D , Ingen-Housz-Oro S , et al. Prognostic factors of paraneoplastic pemphigus[J]. Arch Dermatol, 2012, 148 (10): 1165.
doi: 10.1001/archdermatol.2012.1830 |
| 16 |
Dong Y , Wang M , Nong L , et al. Clinical and laboratory characterization of 114 cases of Castleman disease patients from a single centre: paraneoplastic pemphigus is an unfavourable prognostic factor[J]. Br J Haematol, 2015, 169 (6): 834- 842.
doi: 10.1111/bjh.13378 |
| 17 |
Ouedraogo E , Gottlieb J , de Masson A , et al. Risk factors for death and survival in paraneoplastic pemphigus associated with hematologic malignancies in adults[J]. J Am Acad Dermatol, 2019, 80 (6): 1544- 1549.
doi: 10.1016/j.jaad.2018.03.043 |
| 18 | Wang M , Li F , Wang X , et al. Features and risk factors for paraneoplastic autoimmune multiorgan syndrome in 145 Chinese patients[J]. Acta Derm Venereol, 2020, 100 (18): adv00312. |
| 19 | Kartan S , Shi VY , Clark AK , et al. Paraneoplastic pemphigus and autoimmune blistering diseases associated with neoplasm: characteristics, diagnosis, associated neoplasms, proposed pathogenesis, treatment[J]. Am J Clin Dermatol, 2017, 18 (1): 105- 126. |
| [1] | 周广平,周倩云,朱继红. TAFRO综合征1例[J]. 北京大学学报(医学版), 2021, 53(4): 814-817. |
| Viewed | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
Full text 126
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
Abstract 1061
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||
Cited |
|
|||||||||||||||||||||||||||||||||||||||||||||||||
| Shared | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Discussed | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
||