北京大学学报(医学版) ›› 2026, Vol. 58 ›› Issue (1): 10-21. doi: 10.19723/j.issn.1671-167X.2026.01.002
miR-488-5p促进大鼠骨髓间充质干细胞成神经、成骨分化及神经化骨再生
曾立婷1, 程凯远1,2, 刘中宁1, 李健1, 杨静文1,*( ), 姜婷1,*(
), 姜婷1,*( )
)
- 1. 北京大学口腔医学院·口腔医院修复科, 国家口腔医学中心, 国家口腔疾病临床医学研究中心, 口腔生物材料和数字诊疗装备国家工程研究中心, 北京 100081
2. 天津市口腔医院修复一科, 南开大学医学院, 天津市口腔功能重建重点实验室, 天津 300041
miR-488-5p promotes osteogenic and neurogenic differentiation of rat bone marrow mesenchymal stem cells and enhances neuralized bone regeneration
Liting ZENG1, Kaiyuan CHENG1,2, Zhongning LIU1, Jian LI1, Jingwen YANG1,*( ), Ting JIANG1,*(
), Ting JIANG1,*( )
)
- 1. Department of Prosthodontics, Peking University School and Hospital of Stomatology & National Center for Stomatology & National Clinical Research Center for Oral Diseases & National Engineering Research Center of Oral Biomaterials and Digital Medical Devices, Beijing 100081, China
2. Department Ⅰ of Prosthodontics, Tianjin Stomatological Hospital, School of Medicine, Nankai University, Tianjin Key Laboratory of Oral and Maxillofacial Function Reconstruction, Tianjin 300041, China
摘要:
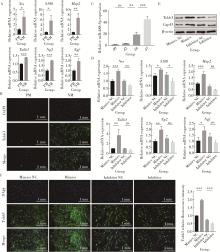
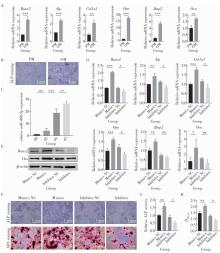
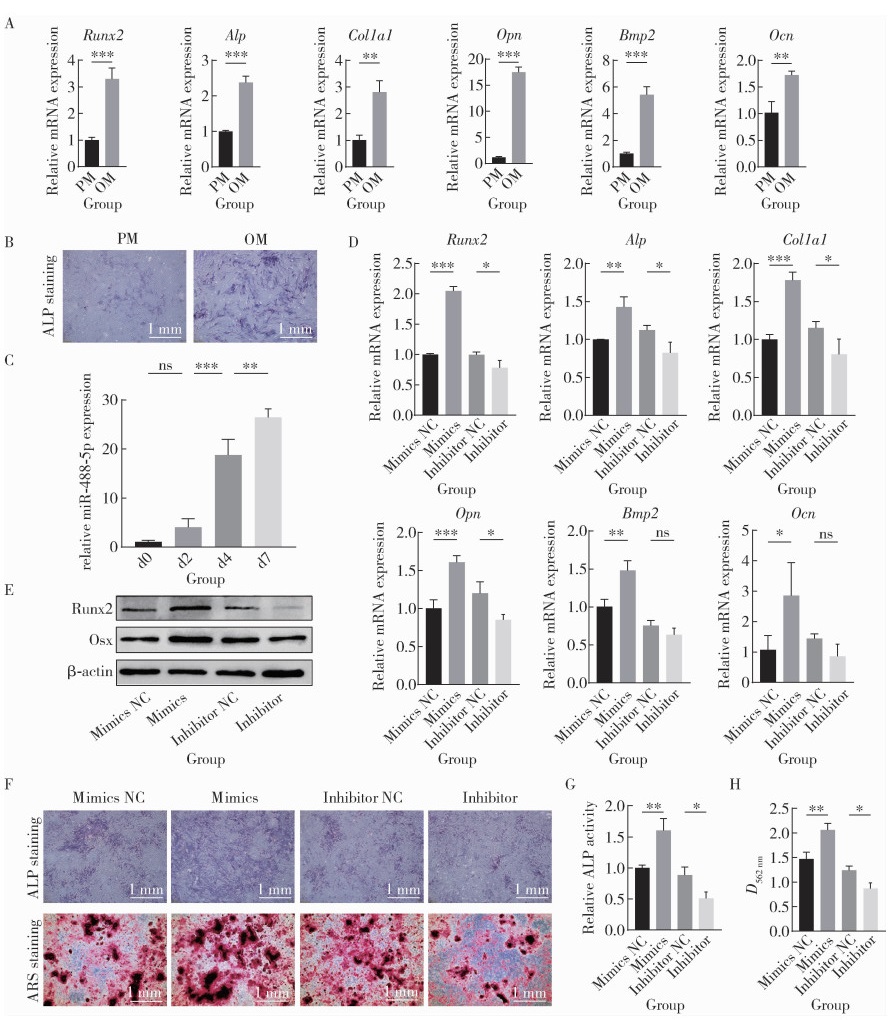
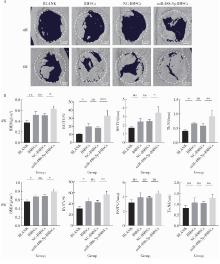
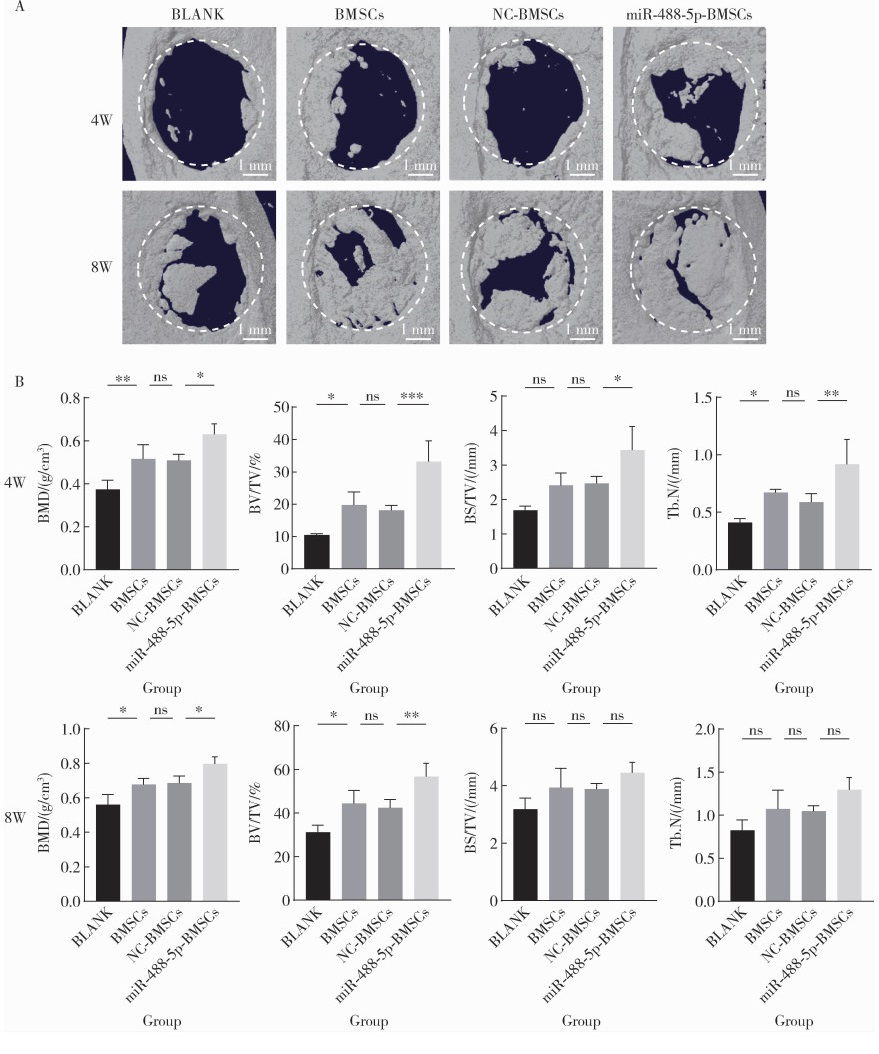
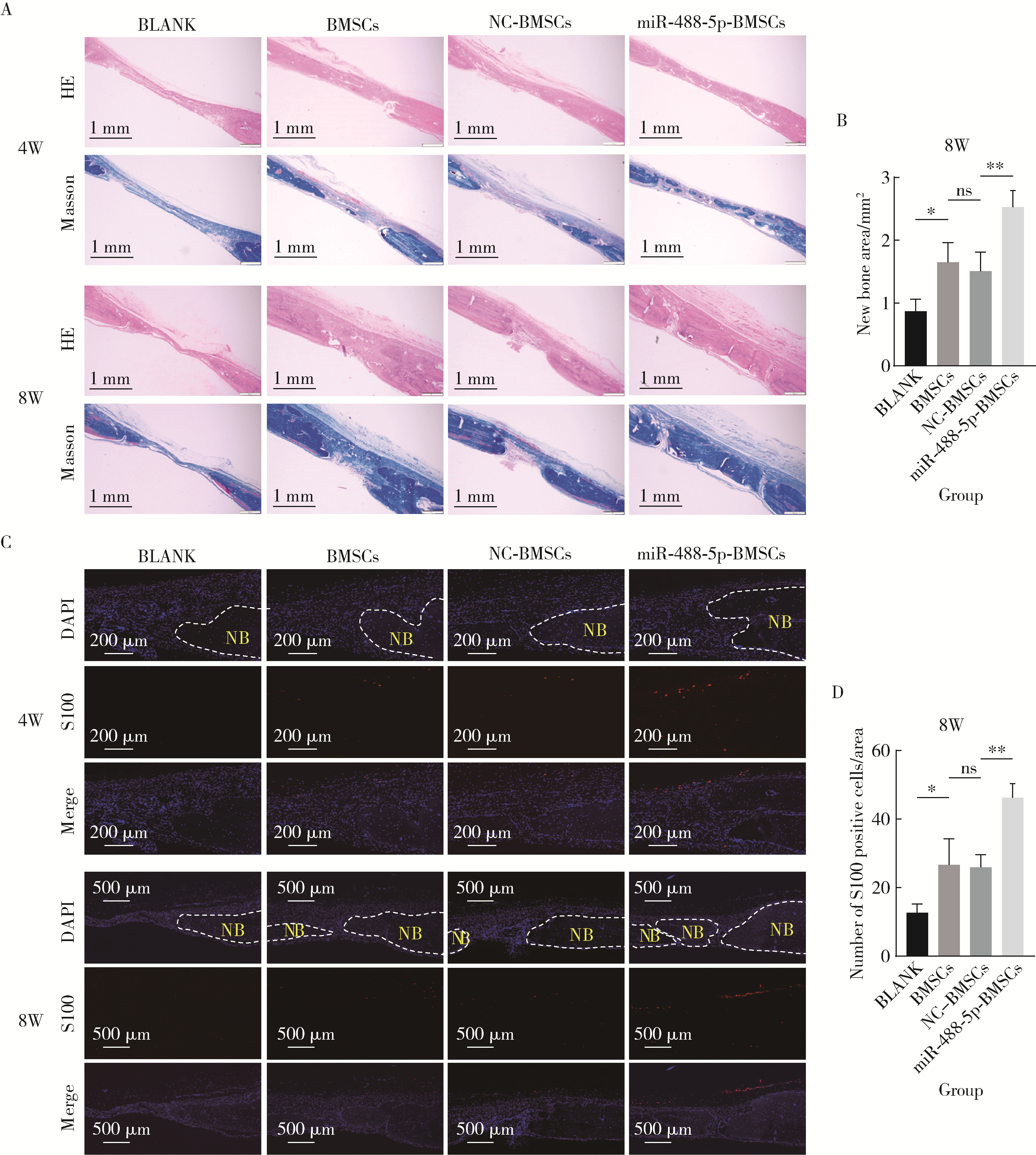
目的: 探讨miR-488-5p促进大鼠骨髓间充质干细胞(rat bone marrow mesenchymal stem cells, rBMSCs)成神经或成骨分化的作用, 以及对神经化骨再生的影响。方法: 体外诱导rBMSCs向成神经或成骨向分化, 实时荧光定量聚合酶链反应(quantitative real-time polymerase chain reaction, qRT-PCR)检测成神经或成骨分化过程中不同时间点(第0、2、4、7天)miR-488-5p的表达水平。建立miR-488-5p模拟物组、miR-488-5p抑制剂组以及各自的阴性对照组(negative control, NC)共四组, 分别探讨miR-488-5p对rBMSCs神经向分化及成骨向分化的影响。构建大鼠颅骨缺损模型(直径5 mm全层圆形临界骨缺损), 光固化甲基丙烯酰化明胶(gelatin methacryloyl, GelMA)负载rBMSCs形成以下四组并进行体内成骨实验: ①BLANK组: GelMA; ②BMSCs组: GelMA+rBMSCs; ③NC-BMSCs组: GelMA+转染miR-488-5p模拟物NC的rBMSCs; ④miR-488-5p-BMSCs组: GelMA+转染miR-488-5p模拟物的rBMSCs。术后4周和8周获取标本, 进行显微CT扫描及影像学骨参数分析, 并通过苏木精-伊红染色、Masson染色和神经特异性蛋白可溶性蛋白100(soluble protein-100, S100)组织免疫荧光染色, 评估缺损区神经化组织工程骨的生成效果。结果: 成功诱导rBMSCs向成神经或成骨向分化, 在rBMSCs成神经或成骨向分化过程中, miR-488-5p从第0天至第7天表达水平均显著升高。给予干预后, rBMSCs的模拟物组成神经相关基因及蛋白表达较其对照组增高, 抑制剂组则呈相反结果; 模拟物组的成骨相关基因及蛋白表达较其对照组增高, 碱性磷酸梅(alkaline phosphatase, ALP)染色及茜素红染色加深, ALP活性增强, 抑制剂组则呈相反结果。骨缺损动物模型结果显示, 术后4周和8周, BLANK组新生骨量最少, BMSCs组和NC-BMSCs组新生骨量居中且相近, miR-488-5p-BMSCs组新生骨量最多, 4周时miR-488-5p-BMSCs组骨矿物质密度[(0.63±0.05) g/cm3 vs. (0.51±0.03) g/cm3]、骨体积分数(33.17%±6.43% vs. 18.11%±1.52%)、骨表面积密度[(3.43±0.69) /mm vs. (2.46±0.20) /mm]及骨小梁数量[(0.92±0.21) /mm vs.(0.59±0.07) /mm]显著高于NC-BMSCs组, 8周时miR-488-5p-BMSCs组的骨矿物质密度[(0.80±0.04) g/cm3 vs. (0.68±0.04) g/cm3]、骨体积分数(56.69%±6.22% vs. 42.36%±3.86%)及新生骨周围被S100标记的神经细胞数量(46.33±4.04 vs. 26.00±3.61)显著高于NC-BMSCs组。结论: miR-488-5p同时具有促进rBMSCs成神经及成骨分化的作用, 并促进大鼠颅骨缺损神经化组织工程骨的形成。
中图分类号:
- R68
| 1 |
doi: 10.1038/s41413-023-00302-8 |
| 2 |
|
| 3 |
doi: 10.1021/acsami.4c16786 |
| 4 |
|
| 5 |
doi: 10.1186/s13287-021-02433-3 |
| 6 |
doi: 10.1016/j.biomaterials.2021.120995 |
| 7 |
doi: 10.1002/advs.202206155 |
| 8 |
|
| 9 |
doi: 10.1016/j.mtbio.2024.100985 |
| 10 |
doi: 10.1111/nyas.13206 |
| 11 |
doi: 10.3390/ijms16048227 |
| 12 |
doi: 10.1002/jbm4.10213 |
| 13 |
doi: 10.1016/j.addr.2015.05.007 |
| 14 |
doi: 10.1002/jnr.25181 |
| 15 |
doi: 10.3390/ijms21041252 |
| 16 |
doi: 10.1111/nyas.14120 |
| 17 |
程凯远, 张美子, 刘中宁, 等. 下牙槽神经离断对下颌骨骨质和拔牙创愈合的影响研究[J]. 口腔颌面修复学杂志, 2025, 26(2): 105- 115.
|
| 18 |
doi: 10.1002/advs.202003390 |
| 19 |
doi: 10.1371/journal.pone.0044768 |
| 20 |
doi: 10.1039/C9TB00025A |
| 21 |
|
| 22 |
|
| 23 |
doi: 10.1007/s11033-024-09577-4 |
| 24 |
doi: 10.1159/000095985 |
| 25 |
doi: 10.1016/j.biomaterials.2021.121216 |
| 26 |
doi: 10.3390/polym16213039 |
| 27 |
doi: 10.1186/s40824-023-00422-6 |
| 28 |
doi: 10.1021/acs.biomac.3c00279 |
| 29 |
丁梦, 李强, 李筱叶, 等. 光固化水凝胶负载骨髓间充质干细胞修复大鼠颅骨缺损[J]. 口腔疾病防治, 2024, 32(5): 330- 340.
|
| 30 |
doi: 10.1016/j.mtbio.2023.100882 |
| 31 |
|
| [1] | 李梦迪, 雷蕾, 刘中宁, 李健, 姜婷. siRNA沉默NLK基因促进神经化组织工程骨再生[J]. 北京大学学报(医学版), 2025, 57(2): 227-236. |
| [2] | 盛春辉, 张晓, 吕珑薇, 周永胜. 人脂肪间充质干细胞外泌体对去势小鼠骨质疏松的预防[J]. 北京大学学报(医学版), 2025, 57(2): 217-226. |
| [3] | 胡轶博, 吕伟佳, 夏炜, 刘亦洪. 基于细胞生长与成骨分化的不同孔径生物支架流体力学有限元分析[J]. 北京大学学报(医学版), 2025, 57(1): 97-105. |
| [4] | 帅婷, 郭艳艳, 林春平, 侯晓玫, 金婵媛. 敲减NPTX1促进人骨髓间充质干细胞成骨分化[J]. 北京大学学报(医学版), 2025, 57(1): 7-12. |
| [5] | 帅婷,刘娟,郭艳艳,金婵媛. 敲减长链非编码RNA MIR4697HG抑制骨髓间充质干细胞成脂向分化[J]. 北京大学学报(医学版), 2022, 54(2): 320-326. |
| [6] | 尤鹏越,刘玉华,王新知,王思雯,唐琳. 脱细胞猪心包膜生物相容性及成骨性能的体内外评价[J]. 北京大学学报(医学版), 2021, 53(4): 776-784. |
| [7] | 谢静,赵玉鸣,饶南荃,汪晓彤,方滕姣子,李晓霞,翟越,李静芝,葛立宏,王媛媛. 3种口腔颌面部来源的间充质干细胞成血管内皮分化潜能的比较研究[J]. 北京大学学报(医学版), 2019, 51(5): 900-906. |
| [8] | 刘霞,李英妮,孙晓麟,彭清林,卢昕,王国春. 去整合素金属蛋白酶对成骨分化的影响[J]. 北京大学学报(医学版), 2018, 50(6): 962-967. |
| [9] | 朱云艳,李倩,张怡美,周彦恒. MAPK和AKT磷酸化下调参与Toll样受体抑制的人牙周膜干细胞的成骨分化[J]. 北京大学学报(医学版), 2018, 50(1): 33-41. |
|
||