北京大学学报(医学版) ›› 2020, Vol. 52 ›› Issue (5): 845-850. doi: 10.19723/j.issn.1671-167X.2020.05.008
不同血清型腺相关病毒载体转染小鼠视网膜后的表达效率
- 北京大学第三医院眼科,北京 100191
Expression pattern of different serotypes of adeno-associated viral vectors in mouse retina
- Department of Ophthalmology, Peking University Third Hospital, Beijing 100191, China
摘要:
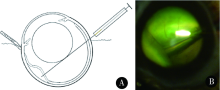
目的:研究不同血清型腺相关病毒 (adeno-associated virus,AAV) 载体介导的外源基因在视网膜中的表达效率, 同时比较AAV载体和两种眼科常用启动子组合后转染小鼠视网膜的表达效率高低,为视网膜色素变性基因治疗选择合适的AAV载体与启动子提供依据。方法:AAV病毒根据衣壳蛋白不同可分为不同血清型,本课题选取视网膜疾病基因治疗中常用的AAV2/2、AAV2/5、AAV2/8和AAV2/9四种血清型AAV载体,并以绿色荧光蛋白 (green fluorescent protein, GFP) 作为报告基因,用GFP的表达强度判断AAV载体介导的外源基因在视网膜中的表达效率。AAV载体纯化后滴度为1.00×10 13 mg/L,注射1 μL至C57BL/6J小鼠视网膜下腔, 于2周取眼球做成冰冻切片,在共聚焦显微镜下观察 GFP在小鼠视网膜各层的表达情况。选取在感光细胞内特异性表达最强的AAV2/8于第4周取眼球冰冻切片继续观察是否能持续稳定表达。随后选取眼科基因治疗最常用的广谱启动子CMV和由CMV增强子与鸡β-肌动蛋白启动子组成的CAG启动子,并构建AAV2/8-GFP-CMV和AAV2/8-GFP-CAG两种不同启动子的病毒载体注射至视网膜下腔,于2周取眼球做成冰冻切片,在共聚焦显微镜下观察不同启动子的AAV2/8在小鼠视网膜各层的表达情况。结果: 注射AAV-GFP后未见典型的术后细菌感染及明显免疫反应。AAV2/2、AAV2/5、AAV2/8和AAV2/9四种血清型AAV载体视网膜下腔注射2周后,AAV2/8和AAV2/9在小鼠视网膜的GFP绿色荧光明显,说明这两种AAV载体转染小鼠视网膜后的表达效率高,而在这两种血清型中,AAV2/8的GFP绿色荧光主要集中在感光细胞内,AAV2/9 在视网膜全层均有表达,说明AAV2/8对视网膜感光细胞特异性更强。对AAV2/8的进一步实验表明在视网膜下腔注射4周后小鼠视网膜的GFP绿色荧光明显,说明AAV2/8载体介导的外源基因能在体内稳定表达。使用CMV启动子时GFP在感光细胞与视网膜色素上皮细胞均有表达,而使用CAG启动子时GFP主要在感光细胞表达。结论:视网膜下腔注射AAV病毒载体可在视网膜细胞内稳定表达报告基因;AAV2/2、AAV2/5、AAV2/8和AAV2/9四种血清型AAV载体中,AAV2/8和AAV2/9在视网膜表达能力最强,AAV2/8对视网膜感光细胞特异性最好;CMV和CAG两种启动子,CAG启动子对感光细胞特异性更高。
中图分类号:
- R774.1
| [1] |
Grieger JC, Choi VW, Samulski RJ. Production and characterization of adeno-associated viral vectors [J]. Nat Protoc, 2006,1(3):1412-1428.
doi: 10.1038/nprot.2006.207 pmid: 17406430 |
| [2] |
Berns KI, Nicholas M. AAV: An overview of unanswered questions[J]. Hum Gene Ther, 2017,28(4):308-313.
doi: 10.1089/hum.2017.048 pmid: 28335618 |
| [3] |
Alves CH, Wijnholds J. AAV gene augmentation therapy for CRB1-associated retinitis pigmentosa[J]. Methods Mol Biol, 2018,1715:135-151.
pmid: 29188511 |
| [4] |
Moore NA, Morral N, Ciulla TA. Gene therapy for inherited retinal and optic nerve degenerations[J]. Expert Opin Biol Ther, 2018,18(1):37-49.
doi: 10.1080/14712598.2018.1389886 pmid: 29057663 |
| [5] |
Sullivan JA, Stanek LM, Lukason MJ. Rationally designed AAV2 and AAVrh8R capsids provide improved transduction in the retina and brain[J]. Gene Ther, 2018,25(3):205-219.
doi: 10.1038/s41434-018-0017-8 pmid: 29785047 |
| [6] |
Ong T, Pennesi ME, Birch DG. Adeno-Associated viral gene therapy for inherited retinal disease[J]. Pharm Res, 2019,36(2):34.
doi: 10.1007/s11095-018-2564-5 pmid: 30617669 |
| [7] |
Russell S, Bennett J, Wellman JA. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: A randomised, controlled, open-label, phase 3 trial[J]. Lancet, 2017,390(10097):849-860.
doi: 10.1016/S0140-6736(17)31868-8 pmid: 28712537 |
| [8] |
Hung SC, Chrysostomou V, Li F. AAV-mediated CRISPR/Cas gene editing of retinal cells in vivo[J]. Invest Ophthalmol Vis Sci, 2016,57(7):3470-3476.
doi: 10.1167/iovs.16-19316 pmid: 27367513 |
| [9] |
Day TP, Byrne LC, Schaffer DV, et al. Advances in AAV vector development for gene therapy in the retina[J]. Adv Exp Med Biol, 2014,801:687-693.
doi: 10.1007/978-1-4614-3209-8_86 |
| [10] |
Allocca M, Mussolino C, Garcia-Hoyos M, et al. Novel Adeno-associated virus serotypes efficiently transduce murine photoreceptors[J]. J Virol, 2007,81(20):11372-11380.
doi: 10.1128/JVI.01327-07 pmid: 17699581 |
| [11] |
Surace EM, Auricchio A. Versatility of AAV vectors for retinal gene transfer[J]. Vision Res, 2008,48(3):353-359.
doi: 10.1016/j.visres.2007.07.027 |
| [12] |
Bennett J, Wellman J, Marshall KA. Safety and durability of effect of contralateral-eye administration of AAV2 gene therapy in patients with childhood-onset blindness caused by RPE65 mutations: a follow-on phase 1 trial[J]. Lancet, 2016,388(10045):661-672.
doi: 10.1016/S0140-6736(16)30371-3 pmid: 27375040 |
| [13] |
Carvalho LS, Xu J, Pearson RA, et al. Long-term and age-dependent restoration of visual function in a mouse model of CNGB3-associated achromatopsia following gene therapy[J]. Hum Mol Genet, 2011,20(16):3161-3175.
doi: 10.1093/hmg/ddr218 |
| [14] |
Flannery JG, Zolotukhin S, Vaquero MI. Efficient photoreceptor-targeted gene expression in vivo by recombinant adeno-associated virus[J]. Proc Natl Acad Sci USA, 1997,94(13):6916-6921.
doi: 10.1073/pnas.94.13.6916 pmid: 9192666 |
| [15] |
Young JE, Vogt T, Gross KW, et al. A short, highly active photoreceptor-specific enhancer/promoter region upstream of the human rhodopsin kinase gene[J]. Invest Ophthalmol Vis Sci, 2003,44(9):4076-4085.
doi: 10.1167/iovs.03-0197 pmid: 12939331 |
| [16] |
Nicoletti A, Kawase K, Thompson DA. Promoter analysis of RPE65, the gene encoding a 61-kDa retinal pigment epithelium-specific protein[J]. Invest Ophthalmol Vis Sci, 1998,39(3):637-644.
pmid: 9501877 |
| [17] |
Esumi N, Oshima Y, Li Y, et al. Analysis of the VMD2 promoter and implication of E-box binding factors in its regulation[J]. J Biol Chem, 2004,279(18):19064-19073.
doi: 10.1074/jbc.M309881200 pmid: 14982938 |
| [18] |
Corti M, Liberati C, Smith BK. Safety of intradiaphragmatic deli-very of adeno-associated virus-mediated alpha-glucosidase (rAAV1-CMV-hGAA) gene therapy in children affected by pompe disease[J]. Hum Gene Ther Clin Dev, 2017,28(4):208-218.
doi: 10.1089/humc.2017.146 pmid: 29160099 |
| [19] |
Martier R, Sogorb-Gonzalez M, Stricker-Shaver J. Development of an AAV-based MicroRNA gene therapy to treat Machado-Joseph disease[J]. Mol Ther Methods Clin Dev, 2019,15:343-358.
doi: 10.1016/j.omtm.2019.10.008 pmid: 31828177 |
| [1] | 吴赤红, 曾争, 王勤环, 于敏, 公维波. 双靶区反义RNA抑制乙型肝炎病毒[J]. 北京大学学报(医学版), 2001, 33(5): 462-464. |
|
||