北京大学学报(医学版) ›› 2022, Vol. 54 ›› Issue (5): 884-895. doi: 10.19723/j.issn.1671-167X.2022.05.016
KRAS G12V特异性T细胞受体治疗恶性肿瘤的临床前研究
程晓静1,蒋栋2,张连海1,王江华2,李雅真2,翟佳慧2,闫宝琪2,张露露2,谢兴旺2,*( ),李子禹1,*(
),李子禹1,*( ),季加孚1,*(
),季加孚1,*( )
)
- 1. 北京大学肿瘤医院暨北京市肿瘤防治研究所胃肠肿瘤中心,恶性肿瘤发病机制及转化研究教育部重点实验室,北京 100142
2. 北京可瑞生物科技有限公司,北京 100094
Preclinical study of T cell receptor specifically reactive with KRAS G12V mutation in the treatment of malignant tumors
Xiao-jing CHENG1,Dong JIANG2,Lian-hai ZHANG1,Jiang-hua WANG2,Ya-zhen LI2,Jia-hui ZHAI2,Bao-qi YAN2,Lu-lu ZHANG2,Xing-wang XIE2,*( ),Zi-yu LI1,*(
),Zi-yu LI1,*( ),Jia-fu JI1,*(
),Jia-fu JI1,*( )
)
- 1. Department of Gastrointestinal Cancer Center, Key Laboratory of Carcinogenesis and Translational Research, Ministry of Education; Laboratory of Genetics, Peking University Cancer Hospital & Institute, Beijing 100142, China
2. Beijing CorreGene Biotechnology Co., Ltd., Beijing 100094, China
摘要:
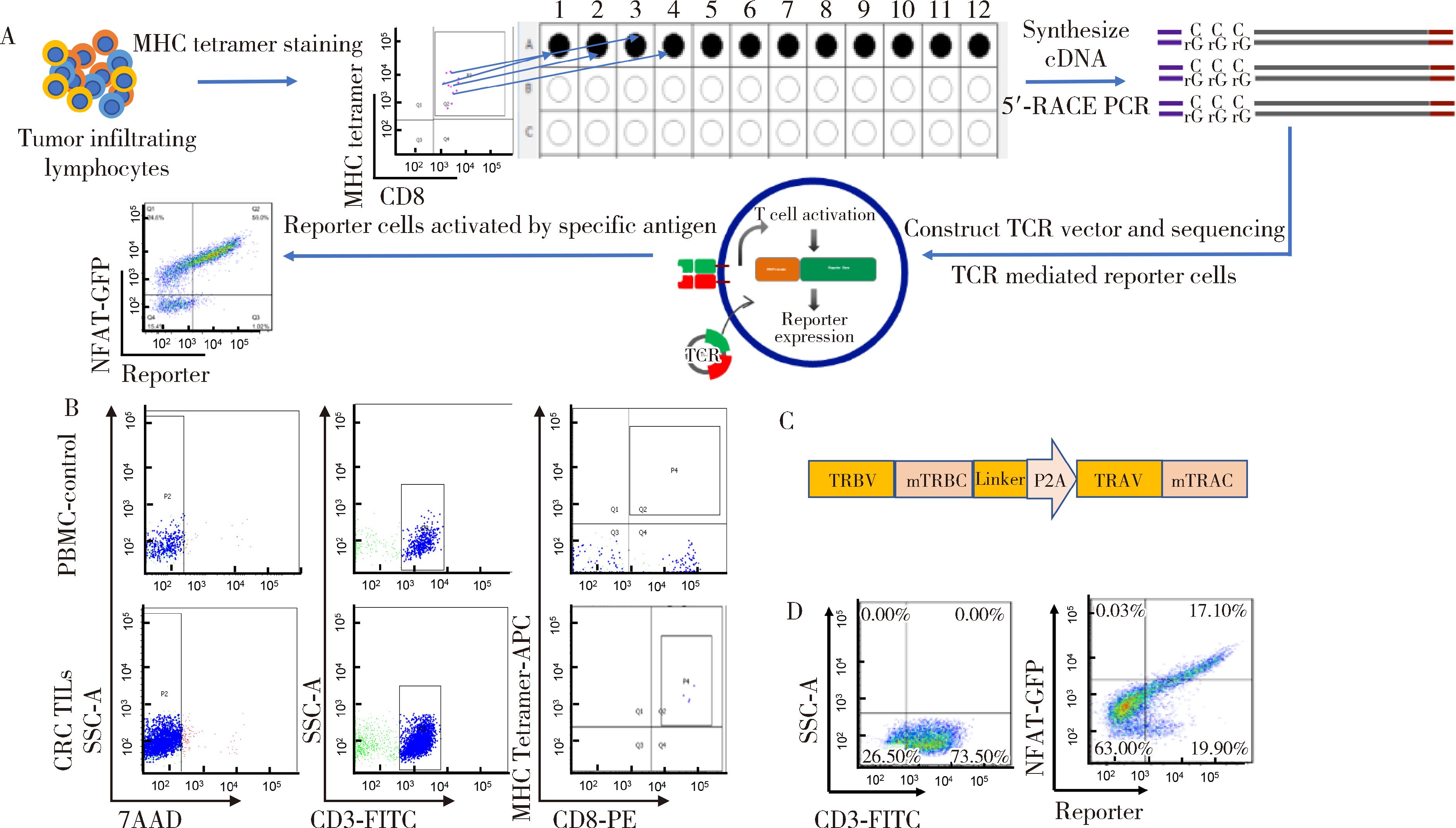
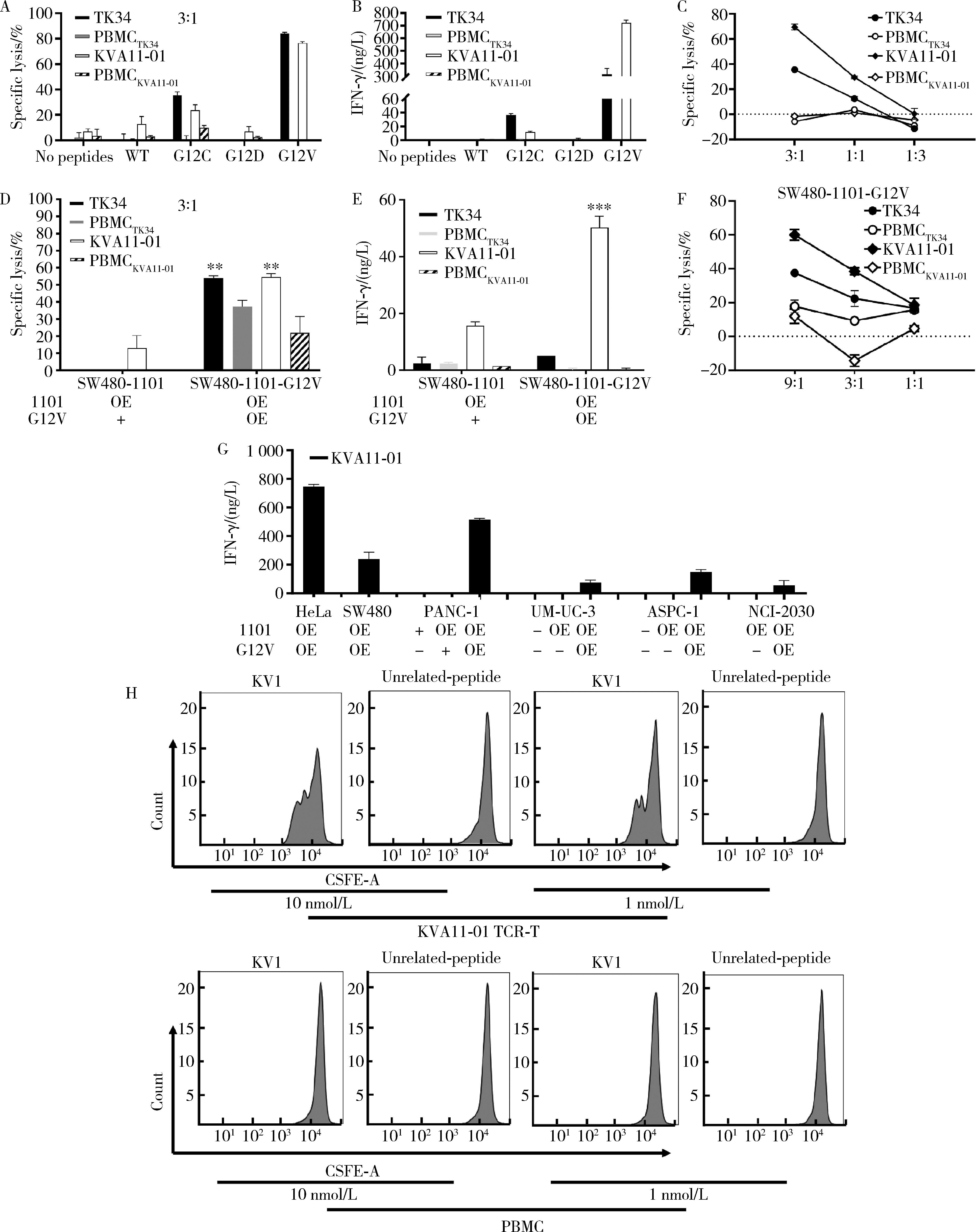
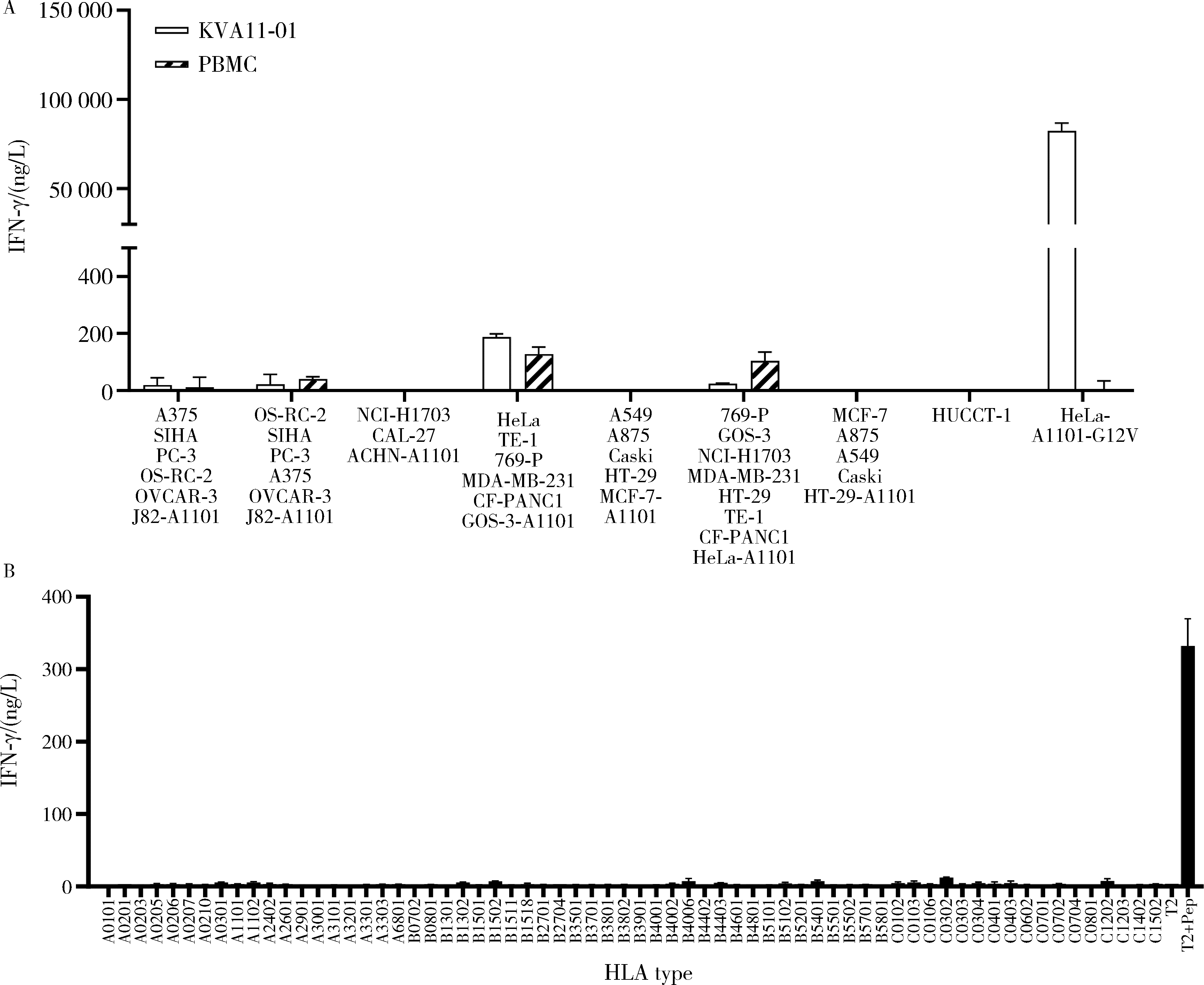
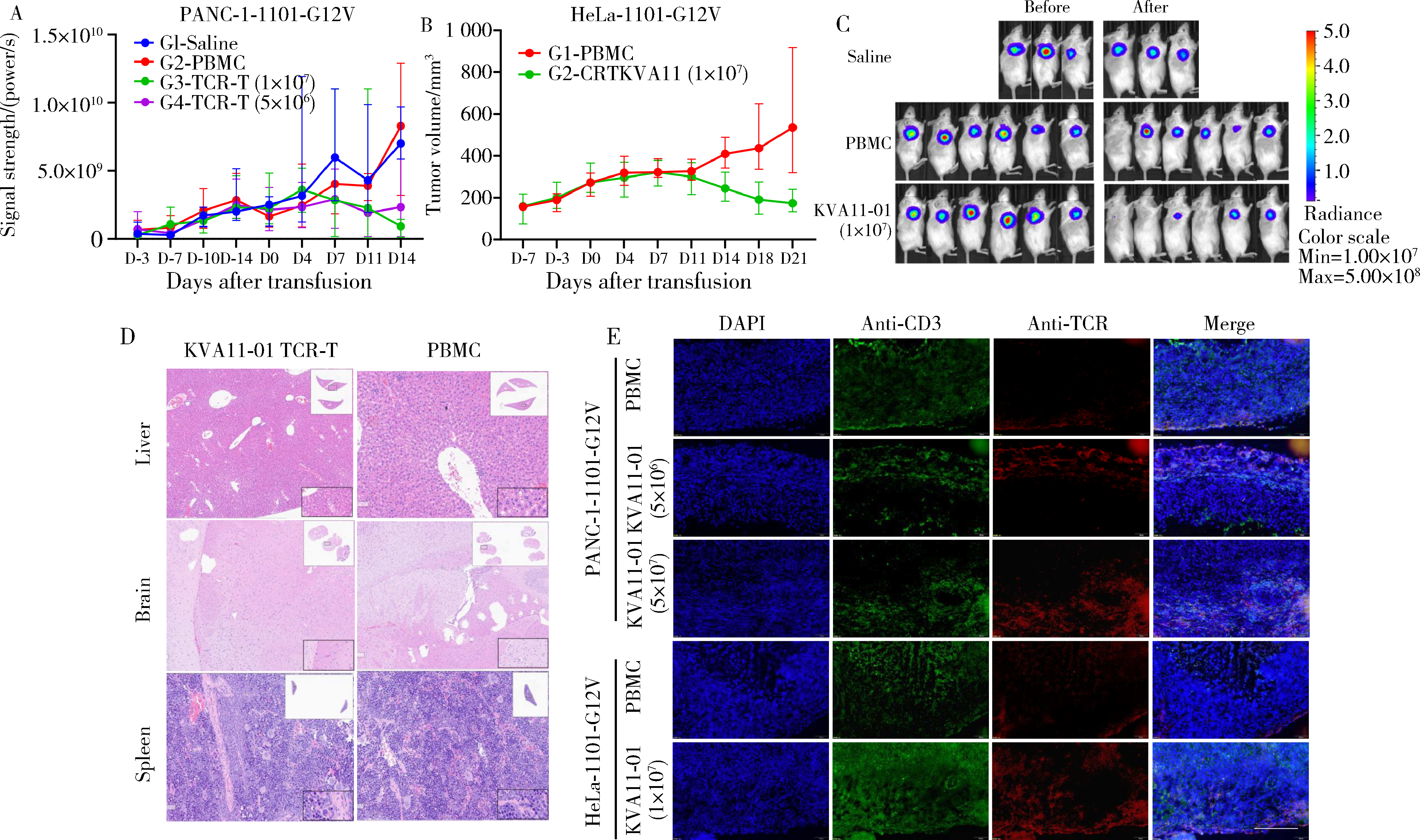
目的: KRAS G12V是最为常见的KRAS突变类型之一,是一个T细胞表位抗原,目前尚无针对该位点的靶向药物,本研究旨在克隆能够特异性识别该表位抗原的T细胞受体(T cell receptor, TCR),通过体内外实验对该TCR基因修饰T细胞(TCR engineered T cells, TCR-T)靶向KRAS G12V突变肿瘤的安全性和有效性进行评估。方法: 从1例结直肠癌患者的肿瘤浸润淋巴细胞中获得靶向KRAS G12V8-16表位的高亲和力TCR序列,构建该TCR慢病毒载体并感染人源T细胞,获得TCR-T。采用抗原肽激活、γ-干扰素(interferon-γ, IFN-γ) 酶联免疫吸附试验(enzyme linked immunosorbent assay,ELISA)、T细胞体外增殖等实验,体外评价该TCR-T的免疫杀伤活性和脱靶-交叉反应性;通过体内实验评价该TCR-T的抑瘤效果、安全性等指标。结果: 获得了能特异性识别HLA-A*11:01限制性KRAS G12V8-16表位的高亲和力TCR序列KVA11-01。KVA11-01 TCR-T能够显著杀伤体外过表达HLA-A*11:01和KRAS G12V的多种肿瘤细胞。非特异杀伤实验显示,KVA11-01仅杀伤同时表达HLA-A*11:01和KRAS G12V的肿瘤细胞。体内抑瘤实验显示,KVA11-01 TCR-T可以显著抑制PANC-1和HeLa(体外过表达HLA-A*11:01和KRAS G12V)细胞裸鼠皮下移植瘤的生长。TCR-T细胞可以显著浸润至肿瘤组织内部,有良好的实体肿瘤归巢能力。结论: KVA11-01 TCR-T能够在体内外有效靶向并杀伤携带KRAS G12V突变的多种恶性肿瘤细胞,具有良好的实体瘤组织归巢能力,KVA11-01 TCR-T有望成为携带KRAS G12V突变的实体恶性肿瘤患者的有效治疗手段。
中图分类号:
- R730.51
| 1 | Bos JL . Ras oncogenes in human cancer: A review[J]. Cancer Res, 1989, 49 (17): 4682- 4689. |
| 2 |
Singh H , Longo DL , Chabner BA . Improving prospects for targeting RAS[J]. J Clin Oncol, 2015, 33 (31): 3650- 3659.
doi: 10.1200/JCO.2015.62.1052 |
| 3 | Hobbs GA , Der CJ , Rossman KL . RAS isoforms and mutations in cancer at a glance[J]. J Cell Sci, 2016, 129 (7): 1287- 1292. |
| 4 |
Windon AL , Loaiza-Bonilla A , Jensen CE , et al. A KRAS wild type mutational status confers a survival advantage in pancreatic ductal adenocarcinoma[J]. J Gastrointest Oncol, 2018, 9 (1): 1- 10.
doi: 10.21037/jgo.2017.10.14 |
| 5 |
Shin SH , Kim SC , Hong SM , et al. Genetic alterations of K-ras, p53, c-erbB-2, and DPC4 in pancreatic ductal adenocarcinoma and their correlation with patient survival[J]. Pancreas, 2013, 42 (2): 216- 222.
doi: 10.1097/MPA.0b013e31825b6ab0 |
| 6 |
Bournet B , Muscari F , Buscail C , et al. KRAS G12D mutation subtype is a prognostic factor for advanced pancreatic adenocarcinoma[J]. Clin Transl Gastroenterol, 2016, 7 (3): e157.
doi: 10.1038/ctg.2016.18 |
| 7 |
Haas M , Ormanns S , Baechmann S , et al. Extended RAS analysis and correlation with overall survival in advanced pancreatic cancer[J]. Br J Cancer, 2017, 116 (11): 1462- 1469.
doi: 10.1038/bjc.2017.115 |
| 8 |
Marabese M , Ganzinelli M , Garassino MC , et al. KRAS mutations affect prognosis of non-small-cell lung cancer patients treated with first-line platinum containing chemotherapy[J]. Oncotarget, 2015, 6 (32): 34014- 34022.
doi: 10.18632/oncotarget.5607 |
| 9 |
Laghi L , Orbetegli O , Bianchi P , et al. Common occurrence of multiple KRAS mutations in pancreatic cancers with associated precursor lesions and in biliary cancers[J]. Oncogene, 2002, 21 (27): 4301- 4306.
doi: 10.1038/sj.onc.1205533 |
| 10 | Russo AL , Borger DR , Szymonifka J , et al. Mutational analysis and clinical correlation of metastatic colorectal cancer[J]. Can-cer, 2014, 120 (10): 1482- 1490. |
| 11 |
Tran E , Ahmadzadeh M , Lu YC , et al. Immunogenicity of soma-tic mutations in human gastrointestinal cancers[J]. Science, 2015, 350 (6266): 1387- 1390.
doi: 10.1126/science.aad1253 |
| 12 |
Chatani PD , Yang JC . Mutated RAS. Targeting the "untargetable" with T cells[J]. Clin Cancer Res, 2020, 26 (3): 537- 544.
doi: 10.1158/1078-0432.CCR-19-2138 |
| 13 |
Tran E , Robbins PF , Lu YC , et al. T-cell transfer therapy targeting mutant KRAS in cancer[J]. N Engl J Med, 2016, 375 (23): 2255- 2262.
doi: 10.1056/NEJMoa1609279 |
| 14 |
Leidner R , Sanjuan Silva N , Huang H , et al. Neoantigen T-cell receptor gene therapy in pancreatic cancer[J]. N Engl J Med, 2022, 386 (22): 2112- 2119.
doi: 10.1056/NEJMoa2119662 |
| 15 | Wang QJ , Yu Z , Griffith K , et al. Identification of T-cell receptors targeting KRAS-mutated human tumors[J]. Cancer Immunol Res, 2015, 4 (3): 204- 214. |
| 16 |
Sim MJW , Lu J , Spencer M , et al. High-affinity oligoclonal TCRs define effective adoptive T cell therapy targeting mutant KRAS-G12D[J]. Proc Natl Acad Sci USA, 2020, 117 (23): 12826- 12835.
doi: 10.1073/pnas.1921964117 |
| 17 |
Cohen CJ , Zhao Y , Zheng Z , et al. Enhanced antitumor activity of murine-human hybrid T-cell receptor (TCR) in human lymphocytes is associated with improved pairing and TCR/CD3 stability[J]. Cancer Res, 2006, 66 (17): 8878- 8886.
doi: 10.1158/0008-5472.CAN-06-1450 |
| 18 |
Morgan RA , Chinnasamy N , Abate-Daga D , et al. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy[J]. J Immunother, 2013, 36 (2): 133- 151.
doi: 10.1097/CJI.0b013e3182829903 |
| 19 |
Linette GP , Stadtmauer EA , Maus MV , et al. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma[J]. Blood, 2013, 122 (6): 863- 871.
doi: 10.1182/blood-2013-03-490565 |
| 20 | Cameron BJ , Gerry AB , Dukes J , et al. Identification of a Titin-derived HLA-A1-presented peptide as a cross-reactive target for engineered MAGE A3-directed T cells[J]. Sci Transl Med, 2013, 5 (197): 103- 126. |
| 21 |
Suchin EJ , Langmuir PB , Palmer E , et al. Quantifying the frequency of alloreactive T cells in vivo: New answers to an old question[J]. J Immunol, 2001, 166 (2): 973- 981.
doi: 10.4049/jimmunol.166.2.973 |
| 22 |
Li J , Li W , Huang K , et al. Chimeric antigen receptor T cell (CAR-T) immunotherapy for solid tumors: lessons learned and strategies for moving forward[J]. J Hematol Oncol, 2018, 11 (1): 22.
doi: 10.1186/s13045-018-0568-6 |
| 23 | Idorn M , Skadborg SK , Kellermann L , et al. Chemokine receptor engineering of T cells with CXCR2 improves homing towards subcutaneous human melanomas in xenograft mouse model[J]. Oncoimmunology, 2018, 7 (8): e1450715. |
| 24 |
Draper LM , Kwong ML , Gros A , et al. Targeting of HPV-16+ epithelial cancer cells by TCR gene engineered T cells directed against E6[J]. Clin Cancer Res, 2015, 21 (19): 4431- 4439.
doi: 10.1158/1078-0432.CCR-14-3341 |
| 25 |
Karapetis CS , Khambata-Ford S , Jonker DJ , et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer[J]. N Engl J Med, 2008, 359 (17): 1757- 1765.
doi: 10.1056/NEJMoa0804385 |
| 26 |
Moore AR , Rosenberg SC , McCormick F , et al. RAS-targeted therapies: Is the undruggable drugged?[J]. Nat Rev Drug Discov, 2020, 19 (8): 533- 552.
doi: 10.1038/s41573-020-0068-6 |
| [1] | 李志存, 吴天俣, 梁磊, 范宇, 孟一森, 张骞. 穿刺活检单针阳性前列腺癌术后病理升级的危险因素分析及列线图模型构建[J]. 北京大学学报(医学版), 2024, 56(5): 896-901. |
| [2] | 刘家骏, 刘国康, 朱玉虎. 免疫相关性重症肺炎1例[J]. 北京大学学报(医学版), 2024, 56(5): 932-937. |
| [3] | 黄教悌,胡菁,韩博. 治疗相关神经内分泌前列腺癌机制研究与靶向治疗新进展[J]. 北京大学学报(医学版), 2024, 56(4): 557-561. |
| [4] | 田宇轩,阮明健,刘毅,李德润,吴静云,沈棋,范宇,金杰. 双参数MRI改良PI-RADS评分4分和5分病灶的最大径对临床有意义前列腺癌的预测效果[J]. 北京大学学报(医学版), 2024, 56(4): 567-574. |
| [5] | 姚凯烽,阮明健,李德润,田宇轩,陈宇珂,范宇,刘毅. 靶向穿刺联合区域系统穿刺对PI-RADS 4~5分患者的前列腺癌诊断效能[J]. 北京大学学报(医学版), 2024, 56(4): 575-581. |
| [6] | 欧俊永,倪坤明,马潞林,王国良,颜野,杨斌,李庚午,宋昊东,陆敏,叶剑飞,张树栋. 肌层浸润性膀胱癌合并中高危前列腺癌患者的预后因素[J]. 北京大学学报(医学版), 2024, 56(4): 582-588. |
| [7] | 王滨帅,邱敏,张前进,田茂锋,刘磊,王国良,陆敏,田晓军,张树栋. 6例肾尤文肉瘤伴静脉瘤栓的诊治[J]. 北京大学学报(医学版), 2024, 56(4): 636-639. |
| [8] | 虞乐,邓绍晖,张帆,颜野,叶剑飞,张树栋. 具有低度恶性潜能的多房囊性肾肿瘤的临床病理特征及预后[J]. 北京大学学报(医学版), 2024, 56(4): 661-666. |
| [9] | 舒帆,郝一昌,张展奕,邓绍晖,张洪宪,刘磊,王国良,田晓军,赵磊,马潞林,张树栋. 肾部分切除术治疗囊性肾癌的功能学和肿瘤学结果:单中心回顾性研究[J]. 北京大学学报(医学版), 2024, 56(4): 667-672. |
| [10] | 方杨毅,李强,黄志高,陆敏,洪锴,张树栋. 睾丸鞘膜高分化乳头状间皮肿瘤1例[J]. 北京大学学报(医学版), 2024, 56(4): 741-744. |
| [11] | 柴晓东,孙子文,李海爽,朱靓怡,刘小旦,刘延涛,裴斐,常青. 髓母细胞瘤分子亚型中CD8+T淋巴细胞浸润的临床病理特点[J]. 北京大学学报(医学版), 2024, 56(3): 512-518. |
| [12] | 林国中,马长城,吴超,司雨,杨军. 微通道技术在颈椎管肿瘤微创切除术中的应用[J]. 北京大学学报(医学版), 2024, 56(2): 318-321. |
| [13] | 俞光岩. 儿童唾液腺疾病[J]. 北京大学学报(医学版), 2024, 56(1): 1-3. |
| [14] | 薛蔚,董樑,钱宏阳,费笑晨. 前列腺癌新辅助治疗与辅助治疗的现状及进展[J]. 北京大学学报(医学版), 2023, 55(5): 775-780. |
| [15] | 薛子璇,唐世英,邱敏,刘承,田晓军,陆敏,董靖晗,马潞林,张树栋. 青年肾肿瘤伴瘤栓的临床病理特征及预后分析[J]. 北京大学学报(医学版), 2023, 55(5): 802-811. |
|
||